Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction
- PMID: 31941367
- PMCID: PMC7122461
- DOI: 10.1161/CIRCULATIONAHA.119.043833
Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction
Abstract
Background: Myocardial infarction (MI) triggers myelopoiesis, resulting in heightened production of neutrophils. However, the mechanisms that sustain their production and recruitment to the injured heart are unclear.
Methods: Using a mouse model of the permanent ligation of the left anterior descending artery and flow cytometry, we first characterized the temporal and spatial effects of MI on different myeloid cell types. We next performed global transcriptome analysis of different cardiac cell types within the infarct to identify the drivers of the acute inflammatory response and the underlying signaling pathways. Using a combination of genetic and pharmacological strategies, we identified the sequelae of events that led to MI-induced myelopoiesis. Cardiac function was assessed by echocardiography. The association of early indexes of neutrophilia with major adverse cardiovascular events was studied in a cohort of patients with acute MI.
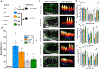
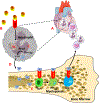
Results: Induction of MI results in rapid recruitment of neutrophils to the infarct, where they release specific alarmins, S100A8 and S100A9. These alarmins bind to the Toll-like receptor 4 and prime the nod-like receptor family pyrin domain-containing 3 inflammasome in naïve neutrophils and promote interleukin-1β secretion. The released interleukin-1β interacts with its receptor (interleukin 1 receptor type 1) on hematopoietic stem and progenitor cells in the bone marrow and stimulates granulopoiesis in a cell-autonomous manner. Genetic or pharmacological strategies aimed at disruption of S100A8/A9 and their downstream signaling cascade suppress MI-induced granulopoiesis and improve cardiac function. Furthermore, in patients with acute coronary syndrome, higher neutrophil count on admission and after revascularization correlates positively with major adverse cardiovascular disease outcomes.
Conclusions: Our study provides novel evidence for the primary role of neutrophil-derived alarmins (S100A8/A9) in dictating the nature of the ensuing inflammatory response after myocardial injury. Therapeutic strategies aimed at disruption of S100A8/A9 signaling or their downstream mediators (eg, nod-like receptor family pyrin domain-containing 3 inflammasome, interleukin-1β) in neutrophils suppress granulopoiesis and may improve cardiac function in patients with acute coronary syndrome.
Keywords: S100A8/A9, Nlrp3 inflammasome; alarmins; inflammation; myelopoiesis; myocardial ischemia; neutrophils.
Figures





Comment in
-
Letter by Wang et al Regarding Article, "Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction".Circulation. 2020 Sep;142(9):e123-e124. doi: 10.1161/CIRCULATIONAHA.120.048102. Epub 2020 Aug 31. Circulation. 2020. PMID: 32866064 No abstract available.
-
Response by Sreejit and Nagareddy to Letter Regarding Article, "Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction".Circulation. 2020 Sep;142(9):e125-e126. doi: 10.1161/CIRCULATIONAHA.120.049408. Epub 2020 Aug 31. Circulation. 2020. PMID: 32866066 Free PMC article. No abstract available.
References
-
- Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost. 2011;106:591–599. doi: 10.1160/TH11-02-0096. - DOI - PubMed
-
- Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Wackers FJ, Jang IK. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–337. doi: 10.1016/j.amjcard.2008.09.085. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

