Early relapse on adjuvant gemcitabine associated with an exceptional response to 2nd line capecitabine chemotherapy in a patient with pancreatic adenosquamous carcinoma with strong intra-tumoural expression of cytidine deaminase: a case report
- PMID: 31941506
- PMCID: PMC6964020
- DOI: 10.1186/s12885-020-6516-1
Early relapse on adjuvant gemcitabine associated with an exceptional response to 2nd line capecitabine chemotherapy in a patient with pancreatic adenosquamous carcinoma with strong intra-tumoural expression of cytidine deaminase: a case report
Abstract
Background: Pancreatic adenosquamous carcinoma has a poor prognosis, with limited prospective trial data to guide optimal treatment. The potential impact of drug metabolism on the treatment response of patients with pancreatic adenosquamous carcinoma is largely unknown.
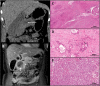
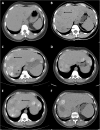
Case presentation: We describe the case of a 51 year old woman with pancreatic adenosquamous carcinoma who, following surgical resection, experienced early disease relapse during adjuvant gemcitabine therapy. Paradoxically, this was followed by an exceptional response to capecitabine therapy lasting 34.6 months. Strong expression of cytidine deaminase was detected within the tumour.
Conclusions: This case study demonstrates that early relapse during adjuvant chemotherapy for pancreatic adenosquamous carcinoma may be compatible with a subsequent exceptional response to second line chemotherapy, an important observation given the poor overall prognosis of patients with adenosquamous carcinoma. Cytidine deaminase is predicted to inactivate gemcitabine and, conversely, catalyze capecitabine activation. We discuss strong intra-tumoural expression of cytidine deaminase as a potential mechanism to explain this patient's disparate responses to gemcitabine and capecitabine therapy, and highlight the benefit that may be gained from considering similar determinants of response to chemotherapy in clinical practice.
Keywords: Cytidine deaminase; Drug metabolism; Gemcitabine; Pancreatic adenosquamous carcinoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- De Souza AL, Saif MW. Platinum-based therapy in adenosquamous pancreatic cancer: experience at two institutions. JOP. 2014;15:144–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

