Dose reduction and withdrawal strategy for TNF-inhibitors in psoriatic arthritis and axial spondyloarthritis: design of a pragmatic open-label, randomised, non-inferiority trial
- PMID: 31941544
- PMCID: PMC6964104
- DOI: 10.1186/s13063-019-4000-5
Dose reduction and withdrawal strategy for TNF-inhibitors in psoriatic arthritis and axial spondyloarthritis: design of a pragmatic open-label, randomised, non-inferiority trial
Abstract
Background: Tumour necrosis factor inhibitors (TNFi) are effective in the treatment of patients with spondyloarthritis (SpA), including psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA). However, these drugs come with some disadvantages such as adverse events, practical burden for patients and high costs. Dose optimisation of TNFi after patients have reached low disease activity (LDA) has been shown feasible and safe in rheumatoid arthritis (RA). However, data on TNFi dose optimisation in PsA and axSpA are scarce, especially pragmatic, randomised strategy studies.
Methods: We developed an investigator-driven, pragmatic, open-label, randomised, controlled, non-inferiority trial (DRESS-PS) to compare the effects of a disease activity-guided treat-to-target strategy with or without a tapering attempt in patients with SpA (PsA and axSpA combined), ≥ 16 years of age, who are being treated with TNFi, and have had at least 6 months of low disease activity. The primary outcome is the percentage of patients in LDA after 12 months of follow up. Patients are assessed at baseline, 3, 6, 9, and 12 months of follow up. Bayesian power analyses with a weakened prior based on a similar study performed in RA resulted in a sample size of 95 patients in total.
Discussion: More knowledge on disease activity-guided treatment algorithms would contribute to better treatment choices and cost savings and potentially decrease the risk of side effects. In this article we elucidate some of our design choices on TNFi dose optimisation and its clinical and methodological consequences.
Trial registration: Dutch Trial Register, NL6771. Registered on 27 November 2018 (CMO NL66181.091.18, 23 October 2018).
Keywords: Dose reduction; Psoriatic arthritis; Randomised controlled trial; Spondyloarthritis; TNF inhibitors; Tapering.
Conflict of interest statement
DvdH has received the following consulting fees: AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma, Director of Imaging Rheumatology bv. AdB has received congress invitations from Roche, Cellgene, Pfizer, Abbvie, Biogen, and expert witness fees from Boehringer, BMS, Amgen, Feseniu, Novartis. The other authors declare that they have no competing interests.
Figures
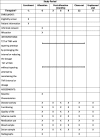
References
-
- Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016;68(2):282–298. doi: 10.1002/art.39298. - DOI - PMC - PubMed
-
- Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. - DOI - PubMed
-
- Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3–17. doi: 10.1136/annrheumdis-2017-211734. - DOI - PMC - PubMed
-
- Ward Michael M., Deodhar Atul, Gensler Lianne S., Dubreuil Maureen, Yu David, Khan Muhammad Asim, Haroon Nigil, Borenstein David, Wang Runsheng, Biehl Ann, Fang Meika A., Louie Grant, Majithia Vikas, Ng Bernard, Bigham Rosemary, Pianin Michael, Shah Amit Aakash, Sullivan Nancy, Turgunbaev Marat, Oristaglio Jeff, Turner Amy, Maksymowych Walter P., Caplan Liron. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis & Rheumatology. 2019;71(10):1599–1613. doi: 10.1002/art.41042. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

