Hemagglutinin and Neuraminidase Antibodies Are Induced in an Age- and Subtype-Dependent Manner after Influenza Virus Infection
- PMID: 31941786
- PMCID: PMC7081922
- DOI: 10.1128/JVI.01385-19
Hemagglutinin and Neuraminidase Antibodies Are Induced in an Age- and Subtype-Dependent Manner after Influenza Virus Infection
Abstract
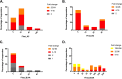
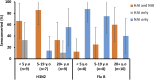
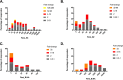
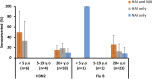
Despite evidence that antibodies targeting the influenza virus neuraminidase (NA) protein can be protective and are broadly cross-reactive, the immune response to NA during infection is poorly understood compared to the response to hemagglutinin (HA) protein. As such, we compared the antibody profile to HA and NA in two naturally infected human cohorts in Auckland, New Zealand: (i) a serosurvey cohort, consisting of pre- and post-influenza season sera from PCR-confirmed influenza cases (n = 50), and (ii) an immunology cohort, consisting of paired sera collected after PCR-confirmation of infection (n = 94). The induction of both HA and NA antibodies in these cohorts was influenced by age and subtype. Seroconversion to HA was more frequent in those <20 years old (yo) for influenza A (serosurvey, P = 0.01; immunology, P = 0.02) but not influenza B virus infection. Seroconversion to NA was not influenced by age or virus type. Adults ≥20 yo infected with influenza A viruses were more likely to show NA-only seroconversion compared to children (56% versus 14% [5 to 19 yo] and 0% [0 to 4 yo], respectively). Conversely, children infected with influenza B viruses were more likely than adults to show NA-only seroconversion (88% [0 to 4 yo] and 75% [5 to 19 yo] versus 40% [≥20 yo]). These data indicate a potential role for immunological memory in the dynamics of HA and NA antibody responses. A better mechanistic understanding of this phenomenon will be critical for any future vaccines aimed at eliciting NA immunity.IMPORTANCE Data on the immunologic responses to neuraminidase (NA) is lacking compared to what is available on hemagglutinin (HA) responses, despite growing evidence that NA immunity can be protective and broadly cross-reactive. Understanding these NA responses during natural infection is key to exploiting these properties for improving influenza vaccines. Using two community-acquired influenza cohorts, we showed that the induction of both HA and NA antibodies after infection is influenced by age and subtypes. Such response dynamics suggest the influence of immunological memory, and understanding how this process is regulated will be critical to any vaccine effort targeting NA immunity.
Keywords: antibody; hemagglutination inhibition; influenza; neuraminidase inhibition; serology.
Copyright © 2020 Wong et al.
Figures
References
-
- Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Nino D, Belmont JW. 2013. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 207:974–981. doi:10.1093/infdis/jis935. - DOI - PMC - PubMed
-
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT Jr, Taubenberger JK. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7:e00417-16. doi:10.1128/mBio.00417-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

