Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains
- PMID: 31941907
- PMCID: PMC6962219
- DOI: 10.1038/s41467-019-14119-9
Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains
Erratum in
-
Author Correction: Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains.Nat Commun. 2020 Mar 9;11(1):1319. doi: 10.1038/s41467-020-15145-8. Nat Commun. 2020. PMID: 32152289 Free PMC article.
Abstract
Chimeric antigen receptor (CAR)-expressing T cells targeting B-cell maturation antigen (BCMA) have activity against multiple myeloma, but improvements in anti-BCMA CARs are needed. We demonstrated recipient anti-CAR T-cell responses against a murine single-chain variable fragment (scFv) used clinically in anti-BCMA CARs. To bypass potential anti-CAR immunogenicity and to reduce CAR binding domain size, here we designed CARs with antigen-recognition domains consisting of only a fully human heavy-chain variable domain without a light-chain domain. A CAR designated FHVH33-CD8BBZ contains a fully human heavy-chain variable domain (FHVH) plus 4-1BB and CD3ζ domains. T cells expressing FHVH33-CD8BBZ exhibit similar cytokine release, degranulation, and mouse tumor eradication as a CAR that is identical except for substitution of a scFv for FHVH33. Inclusion of 4-1BB is critical for reducing activation-induced cell death and promoting survival of T cells expressing FHVH33-containing CARs. Our results indicate that heavy-chain-only anti-BCMA CARs are suitable for evaluation in a clinical trial.
Conflict of interest statement
N.L. is an inventor on a patent application for the FHVH CARs. (PCT/US2018/039917), N.D.T. is an employee of TeneoBio, and is an inventor on patent applications for the heavy-chain-only antibodies (PCT/US2018/038549) and for the FHVH CARs (PCT/US2018/039917). B.B. is an inventor on a patent application for the FHVH CARs (PCT/US2018/039917) and an employee of TeneoBio. J.N.K. is an inventor on a patent application for FHVH CARs (PCT/US2018/039917) and is principle investigator of an anti-BCMA CAR research agreement between the NCI and Celgene.
Figures
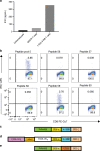
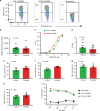
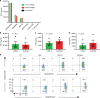

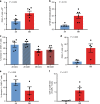

References
-
- Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

