Lipid-based Liquid Crystalline Films and Solutions for the Delivery of Cargo to Cells
- PMID: 31942262
- PMCID: PMC6961842
- DOI: 10.1080/21680396.2019.1666752
Lipid-based Liquid Crystalline Films and Solutions for the Delivery of Cargo to Cells
Abstract
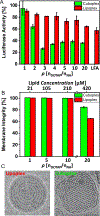
A major challenge in the delivery of cargo (genes and/or drugs) to cells using nanostructured vehicles is the ability to safely penetrate plasma membranes by escaping the endosome before degradation, later releasing the payload into the cytoplasm or organelle of interest. Lipids are a class of bio-compatible molecules that self-assemble into a variety of liquid crystalline constructs. Most of these materials can be used to encapsulate drugs, proteins, and nucleic acids to deliver them safely into various cell types. Lipid phases offer a plethora of structures capable of forming complexes with biomolecules, most notably nucleic acids. The physichochemical characteristics of the lipid molecular building blocks, one might say the lipid primary structure, dictates how they collectively interact to assemble into various secondary structures. These include bilayers, lamellar stacks of bilayers, two-dimensional (2D) hexagonal arrays of lipid tubes, and even 3D cubic constructs. The liquid crystalline materials can be present in the form of aqueous suspensions, bulk materials or confined to a film configuration depending on the intended application (e.g. bolus vs surface-based delivery). This work compiles recent findings of different lipid-based liquid crystalline constructs both in films and particles for gene and drug delivery applications. We explore how lipid primary and secondary structures endow liquid crystalline materials with the ability to carry biomolecular cargo and interact with cells.
Keywords: drug delivery; gene delivery; lipid films; lipid particles; lipid-based liquid crystals; small molecules.
Figures








References
-
- Verkleij AJ. Lipidic intramembranous particles. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1984;779(1):43–63. - PubMed
-
- Yorke M, Dickson D. Lamellar to tubular conformational changes in the endoplasmic reticulum of the retinal pigment epithelium of the newt, notophthalmus viridescens. Cell Tissue Res. 1985;241(3):629–637. - PubMed
-
- Evans DF, Wennerström H. The colloidal domain: Where physics, chemistry, biology, and technology meet. Wiley-Vch New York; 1999.
-
- Radler JO. Structure of DNA-cationic liposome complexes: Dna intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997; 275(5301):810–814. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
