Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy
- PMID: 31943789
- PMCID: PMC7005627
- DOI: 10.15252/emmm.201810154
Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy
Abstract
Diabetic retinopathy (DR) is a common complication of diabetes and leads to blindness. Anti-VEGF is a primary treatment for DR. Its therapeutic effect is limited in non- or poor responders despite frequent injections. By performing a comprehensive analysis of the semaphorins family, we identified the increased expression of Sema4D during oxygen-induced retinopathy (OIR) and streptozotocin (STZ)-induced retinopathy. The levels of soluble Sema4D (sSema4D) were significantly increased in the aqueous fluid of DR patients and correlated negatively with the success of anti-VEGF therapy during clinical follow-up. We found that Sema4D/PlexinB1 induced endothelial cell dysfunction via mDIA1, which was mediated through Src-dependent VE-cadherin dysfunction. Furthermore, genetic disruption of Sema4D/PlexinB1 or intravitreal injection of anti-Sema4D antibody reduced pericyte loss and vascular leakage in STZ model as well as alleviated neovascularization in OIR model. Moreover, anti-Sema4D had a therapeutic advantage over anti-VEGF on pericyte dysfunction. Anti-Sema4D and anti-VEGF also conferred a synergistic therapeutic effect in two DR models. Thus, this study indicates an alternative therapeutic strategy with anti-Sema4D to complement or improve the current treatment of DR.
Keywords: N-cadherin; Sema4D; diabetic retinopathy; mDIA1.
© 2020 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
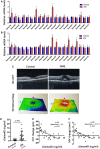
- A
The mRNA levels of semaphorins in age‐matched controls in room air or OIR retinas at P17 were analyzed by qPCR (n = 6 per group, *P < 0.05 compared with room air retinas).
- B
The mRNA levels of semaphorins in retinas 6 months after DM onset were analyzed by qPCR (n = 6 per group, *P < 0.05 compared with vehicle group).
- C
Optical coherence tomography (OCT) and 3D retinal maps from control patients versus DME patients. The purple and light blue lines in the lower panels indicate the scanning level of OCT on the retinas.
- D
The levels of sSEMA4D in the aqueous fluid of DME and control individuals were measured at baseline before initial anti‐VEGF therapy (P < 0.05 compared with control patients).
- E, F
Central subfield thickness (CST) and macular volume (MV) were measured by OCT at baseline before initial anti‐VEGF therapy and 6 months after initial anti‐VEGF therapy. The CST and MV changes were calculated as the baseline value minus the value at 3 months. The correlation curves between the levels of sSema4D in aqueous fluid with changes of CST and MV in response to anti‐VEGF therapy are shown.
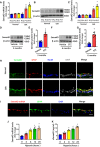
- A
Sema4D mRNA levels in OIR retinas compared with age‐matched controls in room air retinas (n = 6, *P < 0.05 compared with room air retinas).
- B, C
Western blots (B) and quantification (C) of Sema4D protein levels in OIR retinas compared with age‐matched controls in room air retinas (n = 5, *P < 0.05 compared with room air retinas).
- D–G
Western blots (D, F) and quantification (E, G) of Sema4D protein levels in STZ retinas at three and 6 months of diabetes (n = 6 per group, *P < 0.05 compared with vehicle group).
- H
Immunofluorescence staining of Sema4D (green) in OIR retinas with GFAP (red), NeuN (blue), and DAPI (gray) (n = 5), and bars indicate 25 μm.
- I
Fluorescence in situ hybridization with Cy3‐labeled RNA probes targeting Sema4D followed by immunofluorescence staining with GFAP (green) in OIR retinas (n = 5), and scale bars indicate 25 μm.
- J, K
The mRNA levels of Sema4D in cell lysates (J) and secreted protein levels of Sema4D (sSema4D) from conditional medium (K) were increased in primary glial cells after hypoxia (n = 6 per group, *P < 0.05 compared with 0 h group).
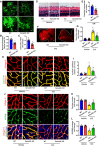
- A–C
Isolectin B4 staining and Evans blue assays were performed to examine pathological retinal neovascularization and vascular leakage in whole‐mount retinas at P17 in the OIR model with or without Sema4D. For isolectin B4 staining, multiple overlapping (10–20% overlap) images were obtained with a 4× lens on a fluorescence microscope. The images were merged to visualize the entire retinas. The upper images are representative composite images of entire retinas. The lower images are a cropped enlarged view from the upper entire retina images. The processes were used in all the following isolectin B4 staining figures for the entire retina images (n = 12 for WT in B, n = 13 for Sema4D‐KO in B, n = 10 in C, scale bars indicate 200 μm for upper pictures and 100 μm for lower pictures, *P < 0.05 compared with WT group).
- D, E
Representative HE staining images and quantification of pre‐retinal neovascular cells in retinal cross‐sections at P17 in the OIR model with or without Sema4D (n = 10. Arrows indicate pre‐retinal neovascular cells. Scale bars indicate 20 μm, *P < 0.05 compared with WT group).
- F, G
Evans blue assays were used to test vascular leakage at 3 months in the STZ model with or without Sema4D. Representative Evans blue fluorescent images are shown. The calculated extracted Evans blue values were used for quantification (n = 12, scale bars indicate 200 μm, *P < 0.05 compared with WT + vehicle group, # P < 0.05 compared with WT + STZ group).
- H, I
Immunofluorescence staining of isolectin B4 (green) with collagen IV (red) showed that Sema4D knockout attenuated acellular capillary formation at 3 months in the STZ model (n = 6. Arrows indicate acellular capillaries. Scale bars indicate 10 μm, *P < 0.05 compared with WT + vehicle group, # P < 0.05 compared with WT + STZ group).
- J–L
Immunofluorescence staining of PDGFRβ (green, a pericyte marker) with collagen IV (red) at 3 months in the STZ model (n = 6, scale bars indicate 10 μm, *P < 0.05 compared with WT + vehicle group, # P < 0.05 compared with WT + STZ group).
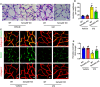
- A, B
Retinal trypsin digestion showed that Sema4D knockout attenuated acellular capillary formation at 3 months in the STZ model (n = 6. Arrows indicate acellular capillaries. Scale bars indicate 20 μm, *P < 0.05 compared with WT + Vehicle group, # P < 0.05 compared with WT + STZ group).
- C, D
Immunofluorescence staining of desmin (green, a pericyte marker) with collagen IV (red) at 3 months in the STZ model with or without Sema4D (n = 6, scale bars indicate 10 μm, *P < 0.05 compared with WT + Vehicle group, # P < 0.05 compared with WT + STZ group).
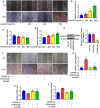
- A, B
Wound‐healing assays indicated that recombinant Sema4D promoted endothelial cell migration in a dose‐dependent manner (n = 5. The vertical red lines indicate the border of the wound. Scale bars indicate 100 μm, *P < 0.05 compared with 0 ng/ml Sema4D group).
- C, D
Trans‐endothelial electrical resistance (TEER) values and dextran permeability assays showed that recombinant Sema4D promoted endothelial monolayer leakage in a dose‐dependent manner (n = 6 in C, n = 5 in D, *P < 0.05 compared with 0 ng/ml Sema4D group).
- E
Knockdown efficiency of PlexinB1 in endothelial cells transfected with lentivirus‐mediated CRISPR‐plB1 (n = 5, *P < 0.05 compared with CRISPR‐wt group).
- F–I
Endothelial cells transfected with lentivirus‐mediated CRISPR‐wt or CRISPR‐plB1 were treated with or without 1600 ng/ml recombinant Sema4D, and then, wound healing (F and G), TEER value (H), and dextran permeability (I) were measured (n = 5 in G, I. n = 6 in H. The vertical red lines indicate the border of the wound in F. Scale bars indicate 100 μm, *P < 0.05 compared with CRISPR‐wt group, # P < 0.05 compared with CRISPR‐wt + Sema4D group).
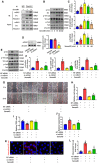
- A
Endothelial cell lysates were prepared and used for an immunoprecipitation assay with anti‐PlexinB1 antibody after the treatment with Sema4D for 30 min. Immunoblots (IB) were performed with antibodies against mDIA1 and Src (n = 4).
- B, C
Western blotting showed that Sema4D induced the phosphorylation of Src, VE‐cadherin, and Fak in endothelial cells in a time‐dependent manner (n = 5, *P < 0.05 compared with 0 min group).
- D
The expression of mDIA1 was silenced by mDIA1 siRNA in endothelial cells (n = 5, *P < 0.05 compared with NT siRNA group).
- E–L
Endothelial cells transfected with NT siRNA or mDIA1 siRNA were treated with or without 1600 ng/ml recombinant Sema4D, followed by Western blotting for the levels of phosphorylation of Src, VE‐cadherin, and Fak after 30‐min treatment (E and F) with specific antibodies. Wound healing (G and H), TEER value (I), dextran permeability (J), and VE‐cadherin internalization (K and L) were measured (n = 5 in F, H, J; n = 6 in I, L. The vertical red lines indicate the border of the wound in G. Scale bars indicate 100 μm for wound healing and 20 μm for VE‐cadherin internalization, *P < 0.05 compared with NT siRNA group, # P < 0.05 compared with NT siRNA + Sema4D group).
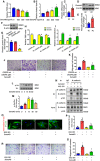
- A, B
The TEER value and dextran permeability were measured in a pericyte (PC) and endothelial cell (EC) co‐culture model after recombinant Sema4D treatment (n = 6 in A, n = 5 in B, *P < 0.05 compared with 0 ng/ml Sema4D group).
- C
The mRNA levels of PlexinB1 in FACS‐sorted endothelial cells and pericytes in retinas (n = 6, *P < 0.05 compared with EC group).
- D, E
Western blots (D) and quantification (E) of PlexinB1 protein levels in cultured pericytes and endothelial cells in vitro (n = 6, *P < 0.05 compared with EC group).
- F, G
Western blots (F) and quantification (G) of PlexinB1 protein levels in the CRISPR‐plB‐mediated knockdown of PlexinB1 in pericytes (n = 5, *P < 0.05 compared with CRISPR‐wt group).
- H, I
Knockdown of PlexinB1 in pericytes reversed Sema4D‐induced permeability to a greater extent than the knockdown of PlexinB1 in endothelial cells in the co‐culture system (n = 6 in H, n = 5 in I, # P < 0.05 compared with CRISPR‐wt + Sema4D group).
- J, K
Transwell migration assays showed that the stimulatory effect of Sema4D on pericyte migration was blocked by PlexinB1 knockdown (n = 6, scale bars indicate 100 μm, *P < 0.05 compared with CRISPR‐wt group, # P < 0.05 compared with CRISPR‐wt + Sema4D group).
- L, M
Western blotting showed that Sema4D induced the phosphorylation of Src in pericytes (n = 5, *P < 0.05 compared with 0 min group).
- N–S
Pericytes pretreated with KX2‐391, an inhibitor of Src, were stimulated with or without 1,600 ng/ml recombinant Sema4D. For N‐cadherin internalization and immunoprecipitation assays, the cells were treated with recombinant Sema4D for 4 h. (N) Cell lysates were used for immunoprecipitation with anti‐N‐cadherin antibody and then immunoblotted with antibodies against β‐catenin and p120‐catenin (n = 4). Pericyte N‐cadherin internalization (O and P) (n = 6, scale bars indicate 20 μm) and migration (R and S) (n = 6, scale bars indicate 100 μm) were evaluated (*P < 0.05 compared with control group, # P < 0.05 compared with Sema4D group).
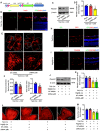
- A
Schematic illustration of the double‐floxed Cre‐inducible GFP and mir30‐shRNA adenovirus (Ad).
- B
GFP staining showed that the shRNA was co‐localized with lectin (red) in OIR retinas (n = 5, scale bars indicate 20 μm).
- C, D
Western blots (C) and quantification (D) of PlexinB1 protein levels confirmed the knockdown efficiency of Ad‐shRNA‐plB1 in OIR retinas (n = 6, *P < 0.05 compared with NT shRNA group).
- E–G
Knockdown of PlexinB1 in endothelial cells reduced pathologic retinal neovascularization and vascular leakage in OIR model. The upper images are representative composite images of entire retinas for isolectin B4. The lower images are a cropped enlarged view from the upper entire retina images. (n = 12 in F, n = 6 in G, scale bars indicate 200 μm for upper pictures and 100 μm for lower pictures, *P < 0.05 compared with NT shRNA group).
- H
GFP staining showed that the shRNA was co‐localized with CD31 (red) in STZ retinas of Tie2‐Cre mice (n = 5, scale bars indicate 20 μm).
- I
GFP staining showed that the shRNA was co‐localized with PDGFRβ (red) in STZ retinas of PDGFRβ‐Cre mice (n = 5, scale bars indicate 20 μm).
- J, K
Western blots (J) and quantification (K) of PlexinB1 protein levels confirmed the knockdown efficiency of Ad‐shRNA‐plB1 in the STZ retinas of Tie2‐Cre mice and PDGFRβ‐Cre mice (n = 6, *P < 0.05 compared with Tie2‐Cre + NT shRNA group, # P < 0.05 compared with PDGFRβ‐Cre + NT shRNA group).
- L, M
Evans blue assays showed that the knockdown of PlexinB1 in endothelial cells or pericytes, respectively, alleviated vascular leakage in the STZ model. Representative Evans blue fluorescent images are shown. The calculated extracted Evans blue values were used for quantification (n = 6, scale bars indicate 200 μm, *P < 0.05 compared with Tie2‐Cre + NT shRNA group, # P < 0.05 compared with PDGFRβ‐Cre + NT shRNA group).
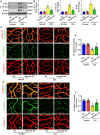
- A, B
Western blotting was performed to detect the phosphorylation of Src, VE‐cadherin, and Fak in whole‐mount retinas at P17 in the normal condition or in the OIR model with or without Sema4D (n = 6, *P < 0.05 compared with WT group in normal condition, # P < 0.05 compared with WT group in OIR).
- C, D
Immunofluorescence staining of VE‐cadherin (green) and collagen IV (red) demonstrated VE‐cadherin continuity in retina at 3 months in the STZ model with or without Sema4D (n = 6, scale bars indicate 10 μm, *P < 0.05 compared with WT + Vehicle group, # P < 0.05 compared with WT + STZ group).
- E, F
Immunofluorescence staining of N‐cadherin (green) and collagen IV (red) demonstrated N‐cadherin coverage in retina at 3 months in the STZ model with or without Sema4D (n = 6, scale bars indicate 10 μm, *P < 0.05 compared with WT + Vehicle group, # P < 0.05 compared with WT + STZ group).

- A, B
In the OIR model, the mice were treated with one single dose of 0.5, 1, or 2 μg anti‐Sema4D (IVI) at P12 and the retinas were harvested at P17. Isolectin B4 staining and Evans blue extraction assays showed a dose‐dependent anti‐Sema4D effect in inhibiting pathological retinal neovascularization and vascular leakage (n = 10 for PBS, IgG, 2 μg anti‐Sema4D in A. n = 12 for 0.5, 1 μg anti‐Sema4D in A. n = 12 in B, *P < 0.05 compared with IgG group).
- C, D
In the OIR model, the WT or Sema4D‐KO mice were treated with one single dose of 2 μg IgG or anti‐Sema4D (IVI) at P12 and the retinas were harvested at P17. Isolectin B4 staining and Evans blue assays were performed (n = 13, 12, 12, 11 in C; n = 12, 11, 13, 12 in D, *P < 0.05 compared with IgG + WT group, NS means no statistical significance).
- E–K
In the OIR model, the mice were treated with one single dose of anti‐Sema4D (2 μg), anti‐VEGF (2 μg) alone or in combination (IVI) at P12 and the retinas were harvested at P17. (E and F) Isolectin B4 staining shows the neovascularization. The upper images are representative composite images of entire retinas. The lower images are a cropped enlarged view from the upper entire retina images. (n = 10, 10, 13, 10, 12 in F; scale bars indicate 200 μm for upper pictures and 100 μm for lower pictures). (G) Evans blue assays were used to quantify the vascular leakage (n = 6). (H and I) Retinal cross‐sections indicated the pre‐retinal neovascular cells (n = 10. Arrows indicate pre‐retinal neovascular cells. Scale bars indicate 20 μm). (J and K) Western blotting detected the phosphorylation of Src, VE‐cadherin, and Fak (n = 5) with the corresponding antibodies (NS means no statistical significance, *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐VEGF group).
- L, M
Evans blue assays showed that one single dose of anti‐Sema4D (2 μg), anti‐VEGF (2 μg) alone, or in combination (IVI.) attenuated pathological vascular leakage 1 week after injection in a 5‐month STZ model. Representative Evans blue fluorescent images are shown. The calculated extracted Evans blue values were used for quantification (n = 6, scale bars indicate 200 μm), NS means no statistical significance, *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐VEGF group.
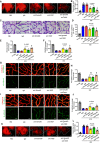
- A, B
Evans blue assays were used to quantify vascular leakage in retinas. Representative Evans blue fluorescent images are shown. The calculated extracted Evans blue values were used for quantification. (n = 12, scale bars indicate 200 μm). *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐Sema4D group.
- C, D
Acellular capillaries were counted after retinal trypsin digestion (n = 6. Arrows indicate acellular capillaries. Scale bars indicate 20 μm). *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐Sema4D group.
- E, F
Quantification of pericyte number and PDGFRβ coverage after immunofluorescence staining of PDGFRβ and collagen IV (n = 6). *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐Sema4D group.
- G, H
Immunofluorescence staining of VE‐cadherin (green) and collagen IV (red) demonstrated VE‐cadherin continuity (n = 6, scale bars indicate 10 μm). *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐Sema4D group.
- I, J
Immunofluorescence staining of N‐cadherin (green) and collagen IV (red) demonstrated N‐cadherin coverage (n = 6, scale bars indicate 10 μm). *P < 0.05 compared with IgG group.
- K, L
The retinas were harvested 2 weeks after the fifth injection, and vascular leakage was quantified by Evans blue assays. Representative Evans blue fluorescent images are shown. The calculated extracted Evans blue values were used for quantification (n = 12, scale bars indicate 200 μm). *P < 0.05 compared with IgG group, # P < 0.05 compared with anti‐Sema4D group.
References
-
- Ashraf M, Souka A, Adelman R (2016) Predicting outcomes to anti‐vascular endothelial growth factor (VEGF) therapy in diabetic macular oedema: a review of the literature. Br J Ophthalmol 100: 1596–1604 - PubMed
-
- Bucher F, Zhang D, Aguilar E, Sakimoto S, Diaz‐Aguilar S, Rosenfeld M, Zha Z, Zhang H, Friedlander M, Yea K (2017) Antibody‐mediated inhibition of Tspan12 ameliorates vasoproliferative retinopathy through suppression of beta‐catenin signaling. Circulation 136: 180–195 - PubMed
-
- Campochiaro PA, Aiello LP, Rosenfeld PJ (2016) Anti‐vascular endothelial growth factor agents in the treatment of retinal disease: from bench to bedside. Ophthalmology 123: S78–S88 - PubMed
-
- Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N, Beaudoin F, Leboeuf D, De Guire V, Binet F et al (2013) Neuron‐derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin‐1. Cell Metab 18: 505–518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2018YFC1312200/National Key R&D Program of China/International
- SATCM-20180339/Major refractory Diseases Pilot Project of Clinical Collaboration with Chinese & Western Medicine/International
- NCET-10-0406/Program for New Century Excellent Talents in University (NCET)/International
- 81601027/National Natural Science Foundation of China/International
- 81820108010/National Natural Science Foundation of China/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

