Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial
- PMID: 31944221
- PMCID: PMC6990720
- DOI: 10.1001/jamaoncol.2019.5868
Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial
Abstract
Importance: A randomized clinical trial is needed to determine whether the second-generation Abl-tyrosine kinase inhibitor dasatinib is more effective than the first-generation inhibitor imatinib mesylate for childhood Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL).
Objective: To determine whether dasatinib given at a daily dosage of 80 mg/m2 is more effective than imatinib mesylate at a daily dosage of 300 mg/m2 to improve event-free survival of children with Philadelphia chromosome-positive ALL in the context of intensive chemotherapy without prophylactic cranial irradiation.
Design, setting, and participants: This open-label, phase 3 randomized clinical trial was conducted at 20 hospitals in China. Enrollment occurred from January 1, 2015, through September 18, 2018, and randomization was stopped on October 4, 2018, when the early stopping criterion of the trial was met. Patients aged 0 to 18 years were recruited. Of the 225 patients with the diagnosis, 35 declined participation and 1 died before treatment, leaving 189 patients available for analysis. Data were analyzed from January 1 through August 4, 2019.
Interventions: Patients were randomized to receive daily dasatinib (n = 92) or imatinib (n = 97) continuously for the entire duration of ALL therapy from the time of diagnosis made during remission induction to the end of continuation therapy.
Main outcomes and measures: The primary outcome was event-free survival, analyzed based on intention to treat. The secondary outcomes were relapse, death due to toxic effects, and overall survival.
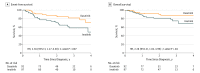
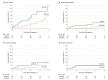
Results: Among the 189 participants (136 male [72.0%]; median age, 7.8 [interquartile range (IQR), 5.2-11.3] years) and a median follow-up of 26.4 (IQR, 16.3-34.1) months, the 4-year event-free survival and overall survival rates were 71.0% (95% CI, 56.2%-89.6%) and 88.4% (95% CI, 81.3%-96.1%), respectively, in the dasatinib group and 48.9% (95% CI, 32.0%-74.5%; P = .005, log-rank test) and 69.2% (95% CI, 55.6%-86.2%; P = .04, log-rank test), respectively, in the imatinib group. The 4-year cumulative risk of any relapse was 19.8% (95% CI, 4.2%-35.4%) in the dasatinib group and 34.4% (95% CI, 15.6%-53.2%) in the imatinib group (P = .01, Gray test), whereas the 4-year cumulative risk of an isolated central nervous system relapse was 2.7% (95% CI, 0.0%-8.1%) in the dasatinib group and 8.4% (95% CI, 1.2%-15.6%) in the imatinib group (P = .06, Gray test). There were no significant differences in the frequency of severe toxic effects between the 2 treatment groups.
Conclusions and relevance: Intensive chemotherapy including dasatinib at a dosage of 80 mg/m2 per day yielded superior results in the treatment of Philadelphia chromosome-positive ALL compared with imatinib mesylate at a dosage of 300 mg/m2 per day and provided excellent control of central nervous system leukemia without the use of prophylactic cranial irradiation.
Trial registration: Chinese Clinical Trial Registry: ChiCTR-IPR-14005706.
Conflict of interest statement
Figures
Comment in
-
Optimizing Targeted Therapy for Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia.JAMA Oncol. 2020 Mar 1;6(3):333-334. doi: 10.1001/jamaoncol.2019.5849. JAMA Oncol. 2020. PMID: 31944218 No abstract available.
-
Dasatinib versus imatinib for childhood acute lymphocytic leukaemia.Lancet Oncol. 2020 Feb;21(2):e73. doi: 10.1016/S1470-2045(20)30031-0. Epub 2020 Jan 23. Lancet Oncol. 2020. PMID: 31982033 No abstract available.
-
Dasatinib versus imatinib in paediatric ALL.Nat Rev Clin Oncol. 2020 Apr;17(4):197. doi: 10.1038/s41571-020-0337-7. Nat Rev Clin Oncol. 2020. PMID: 31996797 No abstract available.
References
-
- Schlieben S, Borkhardt A, Reinisch I, et al. . Incidence and clinical outcome of children with BCR/ABL-positive acute lymphoblastic leukemia (ALL): a prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM-90 and CoALL-05-92. Leukemia. 1996;10(6):957-963. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

