Wood Smoke Particles Stimulate MUC5AC Overproduction by Human Bronchial Epithelial Cells Through TRPA1 and EGFR Signaling
- PMID: 31944254
- PMCID: PMC7098379
- DOI: 10.1093/toxsci/kfaa006
Wood Smoke Particles Stimulate MUC5AC Overproduction by Human Bronchial Epithelial Cells Through TRPA1 and EGFR Signaling
Abstract
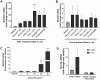
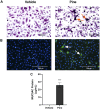
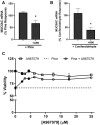
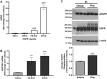
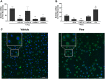
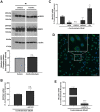
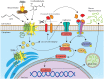
Mucus hypersecretion is a pathological feature of acute inflammatory and chronic obstructive pulmonary diseases. Exposure to air pollutants can be a cause of pathological mucus overproduction, but mechanisms by which different forms of air pollutants elicit this response are not fully understood. In this study, particulate matter (PM) generated from burning pine wood and other types of biomass was used to determine mechanisms by which these forms of PM stimulate mucin gene expression and secretion by primary human bronchial epithelial cells (HBECs). Biomass PM < 2.5 μm generated from pine wood and several other fuels stimulated the expression and secretion of the gel-forming glycoprotein MUC5AC by HBECs. Muc5ac gene induction was also observed in mouse airways following subacute oropharyngeal delivery of pine wood smoke PM. In HBECs, MUC5AC was also induced by the transient receptor potential ankyrin-1 (TRPA1) agonists' coniferaldehyde, a component of pine smoke PM, and allyl isothiocyanate, and was attenuated by a TRPA1 antagonist. Additionally, inhibition of epidermal growth factor receptor (EGFR/ErbB1) and the EGFR signaling partners p38 MAPK and GSK3β also prevented MUC5AC overexpression. Collectively, our results suggest that activation of TRPA1 and EGFR, paired with alterations to p38 MAPK and GSK3β activity, plays a major role in MUC5AC overproduction by bronchial epithelial cells exposed to biomass smoke PM. These results reveal specific processes for how biomass smoke PM may impact the human respiratory system and highlight potential avenues for therapeutic manipulation of lung diseases that are affected by air pollutants.
Keywords: EGFR; MUC5AC; TRPA1; mucin; particulate matter; wood smoke.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Bao Z., Lim S., Liao W., Lin Y., Thiemermann C., Leung B. P., Wong W. (2007). Glycogen synthase kinase-3β inhibition attenuates asthma in mice. Am. J. Respir. Crit. Care Med. 176, 431–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

