High-Aspect-Ratio Nanostructured Surfaces as Biological Metamaterials
- PMID: 31944430
- PMCID: PMC7610849
- DOI: 10.1002/adma.201903862
High-Aspect-Ratio Nanostructured Surfaces as Biological Metamaterials
Abstract
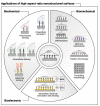
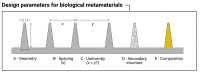
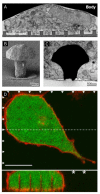
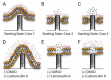
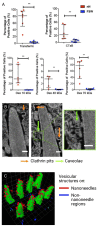
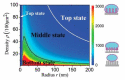
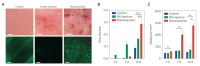
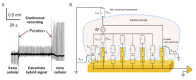
Materials patterned with high-aspect-ratio nanostructures have features on similar length scales to cellular components. These surfaces are an extreme topography on the cellular level and have become useful tools for perturbing and sensing the cellular environment. Motivation comes from the ability of high-aspect-ratio nanostructures to deliver cargoes into cells and tissues, access the intracellular environment, and control cell behavior. These structures directly perturb cells' ability to sense and respond to external forces, influencing cell fate, and enabling new mechanistic studies. Through careful design of their nanoscale structure, these systems act as biological metamaterials, eliciting unusual biological responses. While predominantly used to interface eukaryotic cells, there is growing interest in nonanimal and prokaryotic cell interfacing. Both experimental and theoretical studies have attempted to develop a mechanistic understanding for the observed behaviors, predominantly focusing on the cell-nanostructure interface. This review considers how high-aspect-ratio nanostructured surfaces are used to both stimulate and sense biological systems.
Keywords: biological metamaterials; high-aspect-ratio nanostructures; nanoneedles; nanopillars; nanowires.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















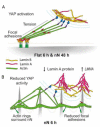
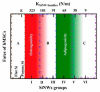

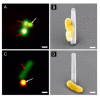

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

