Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR
- PMID: 31945013
- PMCID: PMC7053618
- DOI: 10.18632/aging.102635
Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR
Abstract
Background: It is generally thought that the occurrence and progression of osteoarthritis (OA) results from multiple causes, including degradation and destruction of the cartilage matrix and aging of chondrocytes. Metformin is a first-line drug for the treatment of diabetes, and has great potential for the treatment of other disorders. However, the role of metformin in OA is unknown.
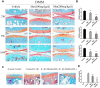
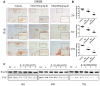
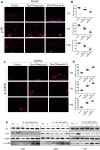
Results: Metformin displayed a protective effect against OA. There were lower OARSI scores and fewer MMP-13-positive cells in DMM mice and cartilage explants after treatment with metformin. In addition, metformin treatment decreased p16INK4a levels in OA chondrocytes, and enhanced polarization of AMPK and inhibition of mTORC1 in OA mice and chondrocytes in a dose-dependent manner.
Conclusions: Metformin effectively alleviated cartilage degradation and aging through regulation of the AMPK/mTOR signaling pathways, suggesting that it could be an effective treatment for OA.
Methods: The effects of metformin on cartilage degradation and chondrocyte aging was determined in a destabilization of the medial meniscus (DMM)-induced OA mouse model and in IL-1β-treated mouse chondrocytes and cartilage explants. Articular cartilage degeneration was graded using the Osteoarthritis Research Society International (OARSI) criteria. Immunostaining, RT-PCR, and western blot analyses were conducted to detect the relative expressions of protein and RNA.
Keywords: AMPK/mTOR; cartilage injury; metformin; osteoarthritis.
Conflict of interest statement
Figures


Similar articles
-
Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis.Ann Rheum Dis. 2015 Jul;74(7):1432-40. doi: 10.1136/annrheumdis-2013-204599. Epub 2014 Mar 20. Ann Rheum Dis. 2015. PMID: 24651621
-
Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway.J Pharmacol Sci. 2017 Jul;134(3):166-174. doi: 10.1016/j.jphs.2017.06.002. Epub 2017 Jun 15. J Pharmacol Sci. 2017. PMID: 28669597
-
Metformin limits osteoarthritis development and progression through activation of AMPK signalling.Ann Rheum Dis. 2020 May;79(5):635-645. doi: 10.1136/annrheumdis-2019-216713. Epub 2020 Mar 10. Ann Rheum Dis. 2020. PMID: 32156705 Free PMC article.
-
The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression.Int J Mol Sci. 2020 Mar 29;21(7):2358. doi: 10.3390/ijms21072358. Int J Mol Sci. 2020. PMID: 32235300 Free PMC article. Review.
-
The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review.Osteoarthritis Cartilage. 2020 Apr;28(4):400-409. doi: 10.1016/j.joca.2020.02.027. Epub 2020 Feb 18. Osteoarthritis Cartilage. 2020. PMID: 32081707 Review.
Cited by
-
The potential benefit of metformin to reduce delirium risk and mortality: a retrospective cohort study.Aging (Albany NY). 2022 Nov 17;14(22):8927-8943. doi: 10.18632/aging.204393. Epub 2022 Nov 17. Aging (Albany NY). 2022. PMID: 36399107 Free PMC article.
-
Metformin Mitigates Cartilage Degradation by Activating AMPK/SIRT1-Mediated Autophagy in a Mouse Osteoarthritis Model.Front Pharmacol. 2020 Jul 24;11:1114. doi: 10.3389/fphar.2020.01114. eCollection 2020. Front Pharmacol. 2020. PMID: 32792951 Free PMC article.
-
Metformin reduces chondrocyte pyroptosis in an osteoarthritis mouse model by inhibiting NLRP3 inflammasome activation.Exp Ther Med. 2022 Mar;23(3):222. doi: 10.3892/etm.2022.11146. Epub 2022 Jan 17. Exp Ther Med. 2022. PMID: 35222699 Free PMC article.
-
Dexamethasone-loaded thermo-sensitive hydrogel attenuates osteoarthritis by protecting cartilage and providing effective pain relief.Ann Transl Med. 2021 Jul;9(14):1120. doi: 10.21037/atm-21-684. Ann Transl Med. 2021. PMID: 34430561 Free PMC article.
-
Research Hotspots and Frontier Trends of Autophagy in Diabetic Cardiomyopathy From 2014 to 2024: A Bibliometric Analysis.J Multidiscip Healthc. 2025 Feb 13;18:837-860. doi: 10.2147/JMDH.S507217. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 39963325 Free PMC article.
References
-
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, et al.. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014; 73:1323–30. 10.1136/annrheumdis-2013-204763 - DOI - PubMed
-
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014; 43:701–12. 10.1016/j.semarthrit.2013.11.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

