Profilin 1 delivery tunes cytoskeletal dynamics toward CNS axon regeneration
- PMID: 31945017
- PMCID: PMC7108904
- DOI: 10.1172/JCI125771
Profilin 1 delivery tunes cytoskeletal dynamics toward CNS axon regeneration
Abstract
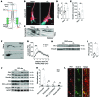
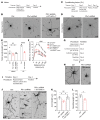
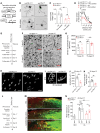
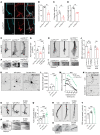
After trauma, regeneration of adult CNS axons is abortive, causing devastating neurologic deficits. Despite progress in rehabilitative care, there is no effective treatment that stimulates axonal growth following injury. Using models with different regenerative capacities, followed by gain- and loss-of-function analysis, we identified profilin 1 (Pfn1) as a coordinator of actin and microtubules (MTs), powering axonal growth and regeneration. In growth cones, Pfn1 increased actin retrograde flow, MT growth speed, and invasion of filopodia by MTs, orchestrating cytoskeletal dynamics toward axonal growth. In vitro, active Pfn1 promoted MT growth in a formin-dependent manner, whereas localization of MTs to growth cone filopodia was facilitated by direct MT binding and interaction with formins. In vivo, Pfn1 ablation limited regeneration of growth-competent axons after sciatic nerve and spinal cord injury. Adeno-associated viral (AAV) delivery of constitutively active Pfn1 to rodents promoted axonal regeneration, neuromuscular junction maturation, and functional recovery of injured sciatic nerves, and increased the ability of regenerating axons to penetrate the inhibitory spinal cord glial scar. Thus, we identify Pfn1 as an important regulator of axonal regeneration and suggest that AAV-mediated delivery of constitutively active Pfn1, together with the identification of modulators of Pfn1 activity, should be considered to treat the injured nervous system.
Keywords: Cell Biology; Cytoskeleton; Neuroscience.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

