A proteomic atlas of senescence-associated secretomes for aging biomarker development
- PMID: 31945054
- PMCID: PMC6964821
- DOI: 10.1371/journal.pbio.3000599
A proteomic atlas of senescence-associated secretomes for aging biomarker development
Abstract
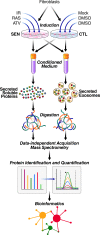
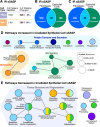
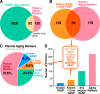
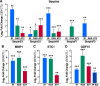
The senescence-associated secretory phenotype (SASP) has recently emerged as a driver of and promising therapeutic target for multiple age-related conditions, ranging from neurodegeneration to cancer. The complexity of the SASP, typically assessed by a few dozen secreted proteins, has been greatly underestimated, and a small set of factors cannot explain the diverse phenotypes it produces in vivo. Here, we present the "SASP Atlas," a comprehensive proteomic database of soluble proteins and exosomal cargo SASP factors originating from multiple senescence inducers and cell types. Each profile consists of hundreds of largely distinct proteins but also includes a subset of proteins elevated in all SASPs. Our analyses identify several candidate biomarkers of cellular senescence that overlap with aging markers in human plasma, including Growth/differentiation factor 15 (GDF15), stanniocalcin 1 (STC1), and serine protease inhibitors (SERPINs), which significantly correlated with age in plasma from a human cohort, the Baltimore Longitudinal Study of Aging (BLSA). Our findings will facilitate the identification of proteins characteristic of senescence-associated phenotypes and catalog potential senescence biomarkers to assess the burden, originating stimulus, and tissue of origin of senescent cells in vivo.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: JC is a founder and shareholder of Unity Biotechnology, which develops senolytic drugs. All other authors have declared no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

