Astrocyte senescence promotes glutamate toxicity in cortical neurons
- PMID: 31945125
- PMCID: PMC6964973
- DOI: 10.1371/journal.pone.0227887
Astrocyte senescence promotes glutamate toxicity in cortical neurons
Abstract
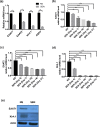
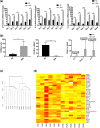
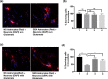
Neurodegeneration is a major age-related pathology. Cognitive decline is characteristic of patients with Alzheimer's and related dementias and cancer patients after chemo- or radio-therapies. A recently emerged driver of these and other age-related pathologies is cellular senescence, a cell fate that entails a permanent cell cycle arrest and pro-inflammatory senescence-associated secretory phenotype (SASP). Although there is a link between inflammation and neurodegenerative diseases, there are many open questions regarding how cellular senescence affects neurodegenerative pathologies. Among the various cell types in the brain, astrocytes are the most abundant. Astrocytes have proliferative capacity and are essential for neuron survival. Here, we investigated the phenotype of primary human astrocytes made senescent by X-irradiation, and identified genes encoding glutamate and potassium transporters as specifically downregulated upon senescence. This down regulation led to neuronal cell death in co-culture assays. Unbiased RNA sequencing of transcripts expressed by non-senescent and senescent astrocytes confirmed that glutamate homeostasis pathway declines upon senescence. Our results suggest a key role for cellular senescence, particularly in astrocytes, in excitotoxicity, which may lead to neurodegeneration including Alzheimer's disease and related dementias.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37(3):653–64. Epub 2003/02/26. 10.1053/jhep.2003.50097 S027091390214208X [pii]. . - DOI - PubMed

