Exploiting azide-alkyne click chemistry in the synthesis, tracking and targeting of platinum anticancer complexes
- PMID: 31945705
- PMCID: PMC7254056
- DOI: 10.1016/j.cbpa.2019.12.001
Exploiting azide-alkyne click chemistry in the synthesis, tracking and targeting of platinum anticancer complexes
Abstract
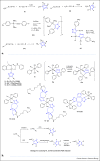
Click chemistry is fundamentally important to medicinal chemistry and chemical biology. It represents a powerful and versatile tool, which can be exploited to develop novel Pt-based anticancer drugs and to better understand the biological effects of Pt-based anticancer drugs at a cellular level. Innovative azide-alkyne cycloaddition-based approaches are being used to functionalise Pt-based complexes with biomolecules to enhance tumour targeting. Valuable information in relation to the mechanisms of action and resistance of Pt-based drugs is also being revealed through click-based detection, isolation and tracking of Pt drug surrogates in biological and cellular environments. Although less well-explored, inorganic Pt-click reactions enable synthesis of novel (potentially multimetallic) Pt complexes and provide plausible routes to introduce functional groups and monitoring Pt-azido drug localisation.
Keywords: Alkyne; Analyse; Anticancer; Azide; CuAAC; Cycloaddition; Functionalise; Platinum; SPAAC; Synthesis; Target; Track; Triazole; iClick.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

