Calcium Phosphate Nanoparticles-Based Systems for RNAi Delivery: Applications in Bone Tissue Regeneration
- PMID: 31947548
- PMCID: PMC7023416
- DOI: 10.3390/nano10010146
Calcium Phosphate Nanoparticles-Based Systems for RNAi Delivery: Applications in Bone Tissue Regeneration
Abstract
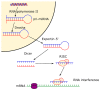
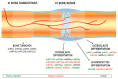
Bone-related injury and disease constitute a significant global burden both socially and economically. Current treatments have many limitations and thus the development of new approaches for bone-related conditions is imperative. Gene therapy is an emerging approach for effective bone repair and regeneration, with notable interest in the use of RNA interference (RNAi) systems to regulate gene expression in the bone microenvironment. Calcium phosphate nanoparticles represent promising materials for use as non-viral vectors for gene therapy in bone tissue engineering applications due to their many favorable properties, including biocompatibility, osteoinductivity, osteoconductivity, and strong affinity for binding to nucleic acids. However, low transfection rates present a significant barrier to their clinical use. This article reviews the benefits of calcium phosphate nanoparticles for RNAi delivery and highlights the role of surface functionalization in increasing calcium phosphate nanoparticles stability, improving cellular uptake and increasing transfection efficiency. Currently, the underlying mechanistic principles relating to these systems and their interplay during in vivo bone formation is not wholly understood. Furthermore, the optimal microRNA targets for particular bone tissue regeneration applications are still unclear. Therefore, further research is required in order to achieve the optimal calcium phosphate nanoparticles-based systems for RNAi delivery for bone tissue regeneration.
Keywords: RNA interference; bone tissue engineering; calcium phosphates; gene therapy; nanoparticles; non-viral vectors; surface functionalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

