Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice
- PMID: 31947657
- PMCID: PMC7016791
- DOI: 10.3390/cells9010210
Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice
Abstract
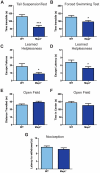
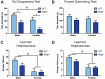
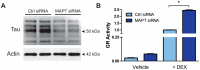
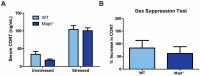
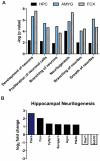
Tau dysfunction is common in several neurodegenerative diseases including Alzheimer's disease (AD) and frontotemporal dementia (FTD). Affective symptoms have often been associated with aberrant tau pathology and are commonly comorbid in patients with tauopathies, indicating a connection between tau functioning and mechanisms of depression. The current study investigated depression-like behavior in Mapt-/- mice, which contain a targeted deletion of the gene coding for tau. We show that 6-month Mapt-/- mice are resistant to depressive behaviors, as evidenced by decreased immobility time in the forced swim and tail suspension tests, as well as increased escape behavior in a learned helplessness task. Since depression has also been linked to deficient adult neurogenesis, we measured neurogenesis in the hippocampal dentate gyrus and subventricular zone using 5-bromo-2-deoxyuridine (BrdU) labeling. We found that neurogenesis is increased in the dentate gyrus of 14-month-old Mapt-/- brains compared to wild type, providing a potential mechanism for their behavioral phenotypes. In addition to the hippocampus, an upregulation of proteins involved in neurogenesis was observed in the frontal cortex and amygdala of the Mapt-/- mice using proteomic mass spectrometry. All together, these findings suggest that tau may have a role in the depressive symptoms observed in many neurodegenerative diseases and identify tau as a potential molecular target for treating depression.
Keywords: Alzheimer’s disease; depression; glucocorticoid receptor; hippocampal neurogenesis; stress; tauopathies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Bierer L.M., Haroutunian V., Gabriel S., Knott P.J., Carlin L.S., Purohit D.P., Perl D.P., Schmeidler J., Kanof P., Davis K.L. Neurochemical Correlates of Dementia Severity in Alzheimer’s Disease: Relative Importance of the Cholinergic Deficits. J. Neurochem. 2002;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

