Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites
- PMID: 31947866
- PMCID: PMC6981555
- DOI: 10.3390/ma13010210
Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites
Abstract
In the development of hydrogen-based technology, a key challenge is the sustainable production of hydrogen in terms of energy consumption and environmental aspects. However, existing methods mainly rely on fossil fuels due to their cost efficiency, and as such, it is difficult to be completely independent of carbon-based technology. Electrochemical hydrogen production is essential, since it has shown the successful generation of hydrogen gas of high purity. Similarly, the photoelectrochemical (PEC) method is also appealing, as this method exhibits highly active and stable water splitting with the help of solar energy. In this article, we review recent developments in PEC water splitting, particularly those using metal-organic halide perovskite materials. We discuss the exceptional optical and electrical characteristics which often dictate PEC performance. We further extend our discussion to the material limit of perovskite under a hydrogen production environment, i.e., that PEC reactions often degrade the contact between the electrode and the electrolyte. Finally, we introduce recent improvements in the stability of a perovskite-based PEC device.
Keywords: metal-organic halide perovskite; passivation; photoelectrochemical reaction; water splitting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
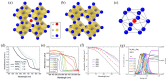





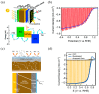
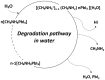



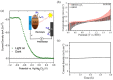
References
-
- UNFCCC The Paris Agreement; Proceedings of the Paris Climate Change Conference, COP 21; Paris, France. 29 January 2016.
-
- Sim U., Moon J., An J., Kang J.H., Jerng S.E., Moon J., Cho S.-P., Hong B.H., Nam K.T. N-Doped Graphene Quantum Sheets on Silicon Nanowire Photocathodes for Hydrogen Production. Energy Environ. Sci. 2015;8:1329–1338. doi: 10.1039/C4EE03607G. - DOI
-
- Sim Y., John J., Surendran S., Moon B., Sim U. Efficient Photoelectrochemical Water Splitting Reaction using Electrodeposited Co3Se4 Catalyst. Appl. Sci. 2019;9:16. doi: 10.3390/app9010016. - DOI
-
- An T.-Y., Surendran S., Kim H., Choe W.-S., Kim J.K., Sim U. A Polydopamine-Mediated Biomimetic Facile Synthesis of Molybdenum Carbide-Phosphide Nanodots Encapsulated in Carbon Shell for Electrochemical Hydrogen Evolution Reaction with Long-Term Durability. Compos. Part B Eng. 2019;175:107071. doi: 10.1016/j.compositesb.2019.107071. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

