Potential and Challenges of Improving Photosynthesis in Algae
- PMID: 31947868
- PMCID: PMC7020468
- DOI: 10.3390/plants9010067
Potential and Challenges of Improving Photosynthesis in Algae
Abstract
Sunlight energy largely exceeds the energy required by anthropic activities, and therefore its exploitation represents a major target in the field of renewable energies. The interest in the mass cultivation of green microalgae has grown in the last decades, as algal biomass could be employed to cover a significant portion of global energy demand. Advantages of microalgal vs. plant biomass production include higher light-use efficiency, efficient carbon capture and the valorization of marginal lands and wastewaters. Realization of this potential requires a decrease of the current production costs, which can be obtained by increasing the productivity of the most common industrial strains, by the identification of factors limiting biomass yield, and by removing bottlenecks, namely through domestication strategies aimed to fill the gap between the theoretical and real productivity of algal cultures. In particular, the light-to-biomass conversion efficiency represents one of the major constraints for achieving a significant improvement of algal cell lines. This review outlines the molecular events of photosynthesis, which regulate the conversion of light into biomass, and discusses how these can be targeted to enhance productivity through mutagenesis, strain selection or genetic engineering. This review highlights the most recent results in the manipulation of the fundamental mechanisms of algal photosynthesis, which revealed that a significant yield enhancement is feasible. Moreover, metabolic engineering of microalgae, focused upon the development of renewable fuel biorefineries, has also drawn attention and resulted in efforts for enhancing productivity of oil or isoprenoids.
Keywords: NPQ; RuBisCO; biomass productivity; complex PSII; light-harvesting; microalgae; photosynthesis; renewable energies; strain domestication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
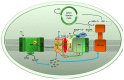

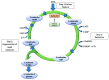
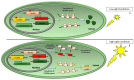
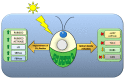
References
-
- Melis A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009;177:272–280. doi: 10.1016/j.plantsci.2009.06.005. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

