A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer
- PMID: 31947974
- PMCID: PMC7016808
- DOI: 10.3390/cancers12010132
A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer
Abstract
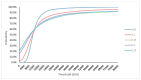
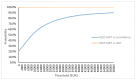
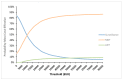
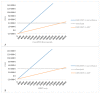
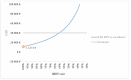
The optimal management of patients with oligorecurrent prostate cancer (PCa) is unknown. There is growing interest in metastasis-directed therapy (MDT) for this population. The objective was to assess cost-utility from a Belgian healthcare payer's perspective of MDT and delayed androgen deprivation therapy (ADT) in comparison with surveillance and delayed ADT, and with immediate ADT. A Markov decision-analytic trial-based model was developed, projecting the results over a 5-year time horizon with one-month cycles. Clinical data were derived from the STOMP trial and literature. Treatment costs were derived from official government documents. Probabilistic sensitivity analyses showed that MDT is cost-effective compared to surveillance (ICER: €8393/quality adjusted life year (QALY)) and immediate ADT (dominant strategy). The ICER is most sensitive to utilities in the different health states and the first month MDT cost. At a willingness-to-pay threshold of €40,000 per QALY, the cost of the first month MDT should not exceed €8136 to be cost-effective compared to surveillance. The Markov-model suggests that MDT for oligorecurrent PCa is potentially cost-effective in comparison with surveillance and delayed ADT, and in comparison with immediate ADT.
Keywords: cost-effective; cost-utility analysis; markov model; metastasis-directed therapy; oligometastasis; oligorecurrent; prostate cancer; prostatic neoplasms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T., Henry A.M., Joniau S., Lam T.B., Mason M.D., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. - DOI - PubMed
-
- Ost P., Reynders D., Decaestecker K., Fonteyne V., Lumen N., De Bruycker A., Lambert B., Delrue L., Bultijnck R., Claeys T., et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. - DOI - PubMed
LinkOut - more resources
Full Text Sources

