TRPC Channels in the SOCE Scenario
- PMID: 31948094
- PMCID: PMC7016597
- DOI: 10.3390/cells9010126
TRPC Channels in the SOCE Scenario
Abstract
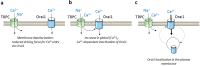
Transient receptor potential (TRP) proteins form non-selective Ca2+ permeable channels that contribute to the modulation of a number of physiological functions in a variety of cell types. Since the identification of TRP proteins in Drosophila, it is well known that these channels are activated by stimuli that induce PIP2 hydrolysis. The canonical TRP (TRPC) channels have long been suggested to be constituents of the store-operated Ca2+ (SOC) channels; however, none of the TRPC channels generate Ca2+ currents that resemble ICRAC. STIM1 and Orai1 have been identified as the components of the Ca2+ release-activated Ca2+ (CRAC) channels and there is a body of evidence supporting that STIM1 is able to gate Orai1 and TRPC1 in order to mediate non-selective cation currents named ISOC. STIM1 has been found to interact to and activate Orai1 and TRPC1 by different mechanisms and the involvement of TRPC1 in store-operated Ca2+ entry requires both STIM1 and Orai1. In addition to the participation of TRPC1 in the ISOC currents, TRPC1 and other TRPC proteins might play a relevant role modulating Orai1 channel function. This review summarizes the functional role of TRPC channels in the STIM1-Orai1 scenario.
Keywords: Orai1; STIM1; TRPC1; calcium influx; store-operated Ca2+ entry (SOCE).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

