Synthesis, Structural Characterization, and Biological Activity of New Pyrazolo[4,3- e][1,2,4]triazine Acyclonucleosides
- PMID: 31948129
- PMCID: PMC6982861
- DOI: 10.3390/molecules25010221
Synthesis, Structural Characterization, and Biological Activity of New Pyrazolo[4,3- e][1,2,4]triazine Acyclonucleosides
Abstract
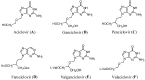
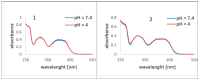
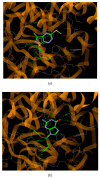
A series of new pyrazolo[4,3-e][1,2,4]triazine acyclonucleosides 2-5 and 8 were prepared and evaluated for their anticancer activity against human cancer cell lines (MCF-7, K-562) and CDK2/E, as well as Abl protein kinases inhibitors. Lipophilicity of the compounds was determined using C-18 and immobilized artificial membrane (IAM) chromatography. In order to confirm the molecular structures and synthesis pathway of new acyclonucleosides, X-ray analysis was performed for model compound 3. Theoretical calculations at the DFT/B3LYP/6-311++G(d,p) level were used for the characterization of electronic structures of 1-8. The potential antiviral activity of acyclonucleosides 2-8 was tested in silico using molecular docking method.
Keywords: X-ray analysis; acyclonucleosides; anticancer activity; molecular docking; pyrazolo[4,3-e][1,2,4]triazine; theoretical calculation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Torii T., Shiragami H., Yamashita K., Suzuki Y., Hijiya T., Kashiwagi T., Izawa K. Practical syntheses of penciclovir and famciclovir from N2-acetyl-7-benzylguanine. Tetrahedron. 2006;62:5709–5716. doi: 10.1016/j.tet.2006.03.080. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

