Natural Compound Library Screening Identifies New Molecules for the Treatment of Cardiac Fibrosis and Diastolic Dysfunction
- PMID: 31948273
- PMCID: PMC7050799
- DOI: 10.1161/CIRCULATIONAHA.119.042559
Natural Compound Library Screening Identifies New Molecules for the Treatment of Cardiac Fibrosis and Diastolic Dysfunction
Abstract
Background: Myocardial fibrosis is a hallmark of cardiac remodeling and functionally involved in heart failure development, a leading cause of deaths worldwide. Clinically, no therapeutic strategy is available that specifically attenuates maladaptive responses of cardiac fibroblasts, the effector cells of fibrosis in the heart. Therefore, our aim was to develop novel antifibrotic therapeutics based on naturally derived substance library screens for the treatment of cardiac fibrosis.
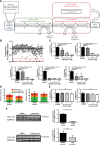
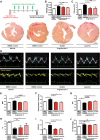
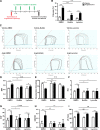
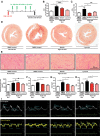
Methods: Antifibrotic drug candidates were identified by functional screening of 480 chemically diverse natural compounds in primary human cardiac fibroblasts, subsequent validation, and mechanistic in vitro and in vivo studies. Hits were analyzed for dose-dependent inhibition of proliferation of human cardiac fibroblasts, modulation of apoptosis, and extracellular matrix expression. In vitro findings were confirmed in vivo with an angiotensin II-mediated murine model of cardiac fibrosis in both preventive and therapeutic settings, as well as in the Dahl salt-sensitive rat model. To investigate the mechanism underlying the antifibrotic potential of the lead compounds, treatment-dependent changes in the noncoding RNAome in primary human cardiac fibroblasts were analyzed by RNA deep sequencing.
Results: High-throughput natural compound library screening identified 15 substances with antiproliferative effects in human cardiac fibroblasts. Using multiple in vitro fibrosis assays and stringent selection algorithms, we identified the steroid bufalin (from Chinese toad venom) and the alkaloid lycorine (from Amaryllidaceae species) to be effective antifibrotic molecules both in vitro and in vivo, leading to improvement in diastolic function in 2 hypertension-dependent rodent models of cardiac fibrosis. Administration at effective doses did not change plasma damage markers or the morphology of kidney and liver, providing the first toxicological safety data. Using next-generation sequencing, we identified the conserved microRNA 671-5p and downstream the antifibrotic selenoprotein P1 as common effectors of the antifibrotic compounds.
Conclusions: We identified the molecules bufalin and lycorine as drug candidates for therapeutic applications in cardiac fibrosis and diastolic dysfunction.
Keywords: diastole; fibrosis; hypertension; microRNAs.
Figures


References
-
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. - PubMed
-
- Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. - PubMed
-
- Heymans S, Gonzalez A, Pizard A, Papageorgiou AP, Lopez-Andres N, Jaisser F, Thum T, Zannad F, Diez J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail. 2015;17:764–771. doi: 10.1002/ejhf.312. - PubMed
-
- Cao Z, Yu D, Fu S, Zhang G, Pan Y, Bao M, Tu J, Shang B, Guo P, Yang P, et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol Lett. 2013;218:174–185. doi: 10.1016/j.toxlet.2013.01.018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

