Iron metabolism and iron disorders revisited in the hepcidin era
- PMID: 31949017
- PMCID: PMC7012465
- DOI: 10.3324/haematol.2019.232124
Iron metabolism and iron disorders revisited in the hepcidin era
Abstract
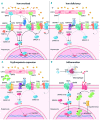
Iron is biologically essential, but also potentially toxic; as such it is tightly controlled at cell and systemic levels to prevent both deficiency and overload. Iron regulatory proteins post-transcriptionally control genes encoding proteins that modulate iron uptake, recycling and storage and are themselves regulated by iron. The master regulator of systemic iron homeostasis is the liver peptide hepcidin, which controls serum iron through degradation of ferroportin in iron-absorptive enterocytes and iron-recycling macrophages. This review emphasizes the most recent findings in iron biology, deregulation of the hepcidin-ferroportin axis in iron disorders and how research results have an impact on clinical disorders. Insufficient hepcidin production is central to iron overload while hepcidin excess leads to iron restriction. Mutations of hemochro-matosis genes result in iron excess by downregulating the liver BMP-SMAD signaling pathway or by causing hepcidin-resistance. In iron-loading anemias, such as β-thalassemia, enhanced albeit ineffective ery-thropoiesis releases erythroferrone, which sequesters BMP receptor ligands, thereby inhibiting hepcidin. In iron-refractory, iron-deficiency ane-mia mutations of the hepcidin inhibitor TMPRSS6 upregulate the BMP-SMAD pathway. Interleukin-6 in acute and chronic inflammation increases hepcidin levels, causing iron-restricted erythropoiesis and ane-mia of inflammation in the presence of iron-replete macrophages. Our improved understanding of iron homeostasis and its regulation is having an impact on the established schedules of oral iron treatment and the choice of oral versus intravenous iron in the management of iron deficiency. Moreover it is leading to the development of targeted therapies for iron overload and inflammation, mainly centered on the manipulation of the hepcidin-ferroportin axis.
Copyright© 2020 Ferrata Storti Foundation.
Figures

References
-
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

