Targeting alkaline ceramidase 3 alleviates the severity of nonalcoholic steatohepatitis by reducing oxidative stress
- PMID: 31949129
- PMCID: PMC6965144
- DOI: 10.1038/s41419-019-2214-9
Targeting alkaline ceramidase 3 alleviates the severity of nonalcoholic steatohepatitis by reducing oxidative stress
Erratum in
-
Correction: Targeting alkaline ceramidase 3 alleviates the severity of nonalcoholic steatohepatitis by reducing oxidative stress.Cell Death Dis. 2020 Mar 17;11(3):191. doi: 10.1038/s41419-020-2396-1. Cell Death Dis. 2020. PMID: 32184395 Free PMC article.
Abstract
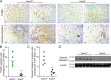
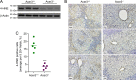
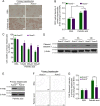
Overload of palmitic acids is linked to the dysregulation of ceramide metabolism in nonalcoholic steatohepatitis (NASH), and ceramides are important bioactive lipids mediating the lipotoxicity of palmitic acid in NASH. However, much remains unclear about the role of ceramidases that catalyze the hydrolysis of ceramides in NASH. By analyzing the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database, we found that alkaline ceramidase 3 (ACER3) is upregulated in livers of patients with NASH. Consistently, we found that Acer3 mRNA levels and its enzymatic activity were also upregulated in mouse livers with NASH induced by a palmitate-enriched Western diet (PEWD). Moreover, we demonstrated that palmitate treatment also elevated Acer3 mRNA levels and its enzymatic activity in mouse primary hepatocytes. In order to investigate the function of Acer3 in NASH, Acer3 null mice and their wild-type littermates were fed a PEWD to induce NASH. Knocking out Acer3 was found to augment PEWD-induced elevation of C18:1-ceramide and alleviate early inflammation and fibrosis but not steatosis in mouse livers with NASH. In addition, Acer3 deficiency attenuated hepatocyte apoptosis in livers with NASH. These protective effects of Acer3 deficiency were found to be associated with suppression of hepatocellular oxidative stress in NASH liver. In vitro studies further revealed that loss of ACER3/Acer3 increased C18:1-ceramide and inhibited apoptosis and oxidative stress in mouse primary hepatocytes and immortalized human hepatocytes induced by palmitic-acid treatment. These results suggest that ACER3 plays an important pathological role in NASH by mediating palmitic-acid-induced oxidative stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

