Pharmacogenomic associations of adverse drug reactions in asthma: systematic review and research prioritisation
- PMID: 31949291
- PMCID: PMC7502355
- DOI: 10.1038/s41397-019-0140-y
Pharmacogenomic associations of adverse drug reactions in asthma: systematic review and research prioritisation
Erratum in
-
Correction: Pharmacogenomic associations of adverse drug reactions in asthma: systematic review and research prioritization.Pharmacogenomics J. 2020 Oct;20(5):746. doi: 10.1038/s41397-020-0178-x. Pharmacogenomics J. 2020. PMID: 32704026 Free PMC article.
Abstract
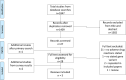
A systematic review of pharmacogenomic studies capturing adverse drug reactions (ADRs) related to asthma medications was undertaken, and a survey of Pharmacogenomics in Childhood Asthma (PiCA) consortia members was conducted. Studies were eligible if genetic polymorphisms were compared with suspected ADR(s) in a patient with asthma, as either a primary or secondary outcome. Five studies met the inclusion criteria. The ADRs and polymorphisms identified were change in lung function tests (rs1042713), adrenal suppression (rs591118), and decreased bone mineral density (rs6461639) and accretion (rs9896933, rs2074439). Two of these polymorphisms were replicated within the paper, but none had external replication. Priorities from PiCA consortia members (representing 15 institution in eight countries) for future studies were tachycardia (SABA/LABA), adrenal suppression/crisis and growth suppression (corticosteroids), sleep/behaviour disturbances (leukotriene receptor antagonists), and nausea and vomiting (theophylline). Future pharmacogenomic studies in asthma should collect relevant ADR data as well as markers of efficacy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Global Asthma Network. The global asthma report 2014. Auckland, New Zealand: Global Asthma Network; 2014. 769.
-
- World Health Organization. Asthma fact sheet no. 307. 2013. http://www.whoint/topics/asthma/es 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous