Prophylaxis and Therapy of Venous Thrombotic Events (VTE) in Pregnancy and the Postpartum Period
- PMID: 31949319
- PMCID: PMC6957355
- DOI: 10.1055/a-1030-4546
Prophylaxis and Therapy of Venous Thrombotic Events (VTE) in Pregnancy and the Postpartum Period
Abstract
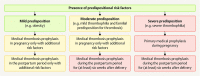
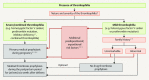
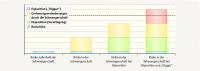
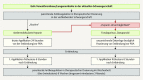
Venous thromboembolisms and pulmonary embolisms are one of the main causes of morbidity and mortality in pregnancy. The increased risk of thrombotic events caused by the physiological changes during pregnancy alone does not justify any medical antithrombotic prophylaxis. However, if there are also other risk factors such as a history of thromboses, hormonal stimulation as part of fertility treatment, thrombophilia, increased age of the pregnant woman, severe obesity or predisposing concomitant illnesses, the risk of thrombosis should be re-evaluated - if possible by a coagulation specialist - and drug prophylaxis should be initiated, where applicable. Low-molecular-weight heparins (LMWH) are the standard medication for the prophylaxis and treatment of thrombotic events in pregnancy and the postpartum period. Medical thrombosis prophylaxis started during pregnancy is generally continued for about six weeks following delivery due to the risk of thrombosis which peaks during the postpartum period. The same applies to therapeutic anticoagulation after the occurrence of a thrombotic event in pregnancy; here, a minimum duration of the therapy of three months should also be adhered to. During breastfeeding, LMWH or the oral anticoagulant warfarin can be considered; neither active substance passes into breast milk.
Keywords: anticoagulation; low-molecular-weight heparins; oral anticoagulants; pregnancy; thromboembolisms.
Conflict of interest statement
Conflict of Interest/Interessenkonflikt Dr. Sucker received payments from pharmaceutical companies that produce parenteral anticoagulants used in pregnancy (Aspen, LeoPharma, Sanofi) and of companies that offer devices and reagents for laboratory analyses on the field of haemostasis (Werfen, Stago). Payments were received for lectures and participation in advisory boards./Dr. Sucker hat von pharmazeutischen Unternehmen, die parenterale Antikoagulanzien zur Anwendung während der Schwangerschaft herstellen (Aspen, LeoPharma, Sanofi), sowie von Unternehmen, die Geräte und Reagenzien für Laboranalysen im Bereich der Hämostase anbieten (Werfen, Stago), Zahlungen für Vorträge und die Mitgliedschaft in Beiräten erhalten.
Figures






Similar articles
-
Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition).Chest. 2008 Jun;133(6 Suppl):844S-886S. doi: 10.1378/chest.08-0761. Chest. 2008. PMID: 18574280
-
Venous thromboembolism and anticoagulant therapy in pregnancy.Gend Med. 2005;2 Suppl A:S10-7. doi: 10.1016/s1550-8579(05)80060-9. Gend Med. 2005. PMID: 16551552 Review.
-
Pregnancy after catheter-directed thrombolysis for acute iliofemoral deep venous thrombosis.Phlebology. 2013 Mar;28 Suppl 1:34-8. doi: 10.1177/0268355513477286. Phlebology. 2013. PMID: 23482532
-
Treatment of pregnancy-associated venous thromboembolism - position paper from the Working Group in Women's Health of the Society of Thrombosis and Haemostasis (GTH).Vasa. 2016;45(2):103-18. doi: 10.1024/0301-1526/a000504. Vasa. 2016. PMID: 27058796 Review.
-
Thromboprophylaxis in pregnant women with thrombophilia and a history of thrombosis.J Perinat Med. 2018 Oct 25;46(8):893-899. doi: 10.1515/jpm-2017-0329. J Perinat Med. 2018. PMID: 29949514
Cited by
-
Fondaparinux Pre-, Peri-, and/or Postpartum for the Prophylaxis/Treatment of Venous Thromboembolism (FondaPPP).Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211014575. doi: 10.1177/10760296211014575. Clin Appl Thromb Hemost. 2021. PMID: 33942675 Free PMC article.
-
Pregnancy and thrombosis risk for women without a history of thrombotic events: a retrospective study of the real risks.Thromb J. 2022 Oct 6;20(1):60. doi: 10.1186/s12959-022-00419-6. Thromb J. 2022. PMID: 36203153 Free PMC article.
-
Serological Parameters and Vascular Investigation for a Better Assessment in DVT during Pregnancy-A Systematic Review.Medicina (Kaunas). 2021 Feb 10;57(2):160. doi: 10.3390/medicina57020160. Medicina (Kaunas). 2021. PMID: 33578903 Free PMC article.
-
Assessment of Saudi Women's Adherence and Experience with Venous Thromboembolism Prophylaxis after Cesarean Section Delivery Using Telemedicine Technology.Appl Bionics Biomech. 2022 Mar 14;2022:8440789. doi: 10.1155/2022/8440789. eCollection 2022. Appl Bionics Biomech. 2022. Retraction in: Appl Bionics Biomech. 2023 Nov 29;2023:9784678. doi: 10.1155/2023/9784678. PMID: 35321355 Free PMC article. Retracted.
-
Diseases and complications of the puerperium.Dtsch Arztebl Int. 2021 Jun 25;118(Forthcoming):436-46. doi: 10.3238/arztebl.m2021.0168. Dtsch Arztebl Int. 2021. PMID: 33972015 Free PMC article. Review.
References
-
- Say L, Chou D, Gemmill A. Global causes of maternal deaths: a WHO systematic analysis. Lancet Glob Health. 2014;2:e329–e338. - PubMed
-
- De Stefano V, Martinelli I, Rossi E. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol. 2006;135:386–391. - PubMed
-
- Gherman R B, Goodwin T M, Leung B. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730–734. - PubMed
-
- Heit J A, Kobbervig C E, James A H. Trends in the incidence of venous thromboembolism during pregnancy and postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697–706. - PubMed
-
- Jacobsen A F, Skjeldestad F E, Sandset P M. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium – a register-based case-control study. Am J Obstet Gynecol. 2008;198:2330–2.33E9. - PubMed
LinkOut - more resources
Full Text Sources

