Regulation and Directing Stem Cell Fate by Tissue Engineering Functional Microenvironments: Scaffold Physical and Chemical Cues
- PMID: 31949436
- PMCID: PMC6948329
- DOI: 10.1155/2019/2180925
Regulation and Directing Stem Cell Fate by Tissue Engineering Functional Microenvironments: Scaffold Physical and Chemical Cues
Abstract
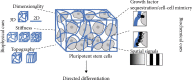
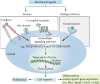
It is well known that stem cells reside within tissue engineering functional microenvironments that physically localize them and direct their stem cell fate. Recent efforts in the development of more complex and engineered scaffold technologies, together with new understanding of stem cell behavior in vitro, have provided a new impetus to study regulation and directing stem cell fate. A variety of tissue engineering technologies have been developed to regulate the fate of stem cells. Traditional methods to change the fate of stem cells are adding growth factors or some signaling pathways. In recent years, many studies have revealed that the geometrical microenvironment played an essential role in regulating the fate of stem cells, and the physical factors of scaffolds including mechanical properties, pore sizes, porosity, surface stiffness, three-dimensional structures, and mechanical stimulation may affect the fate of stem cells. Chemical factors such as cell-adhesive ligands and exogenous growth factors would also regulate the fate of stem cells. Understanding how these physical and chemical cues affect the fate of stem cells is essential for building more complex and controlled scaffolds for directing stem cell fate.
Copyright © 2019 Fei Xing et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Saroia J., Yanen W., Wei Q., Zhang K., Lu T., Zhang B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Design and Manufacturing. 2018;1(4):265–279. doi: 10.1007/s42242-018-0029-7. - DOI
Publication types
LinkOut - more resources
Full Text Sources

