Dropwise condensation on solid hydrophilic surfaces
- PMID: 31950076
- PMCID: PMC6954056
- DOI: 10.1126/sciadv.aax0746
Dropwise condensation on solid hydrophilic surfaces
Abstract
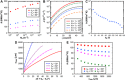
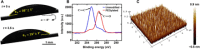
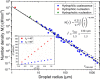
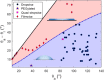
Droplet nucleation and condensation are ubiquitous phenomena in nature and industry. Over the past century, research has shown dropwise condensation heat transfer on nonwetting surfaces to be an order of magnitude higher than filmwise condensation heat transfer on wetting substrates. However, the necessity for nonwetting to achieve dropwise condensation is unclear. This article reports stable dropwise condensation on a smooth, solid, hydrophilic surface (θa = 38°) having low contact angle hysteresis (<3°). We show that the distribution of nano- to micro- to macroscale droplet sizes (about 100 nm to 1 mm) for coalescing droplets agrees well with the classical distribution on hydrophobic surfaces and elucidate that the wettability-governed dropwise-to-filmwise transition is mediated by the departing droplet Bond number. Our findings demonstrate that achieving stable dropwise condensation is not governed by surface intrinsic wettability, as assumed for the past eight decades, but rather, it is dictated by contact angle hysteresis.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Schmidt E., Schurig W., Sellschopp W., Condensation of water vapour in film- and drop form. VDI Z. 74, 544–544 (1930).
-
- Rose J. W., Dropwise condensation theory and experiment: A review. Proc. Inst. Mech. Eng. J. 216, 115–128 (2002).
-
- Alwazzan M., Egab K., Peng B., Khan J., Li C., Condensation on hybrid-patterned copper tubes (I): Characterization of condensation heat transfer. Int. J. Heat Mass Transfer 112, 991–1004 (2017).
-
- Alwazzan M., Egab K., Wang P., Shang Z., Liang X., khan J., Li C., Condensation heat transfer on nickel tubes: The role of atomic layer deposition of nickel oxide. Int. J. Heat Mass Transfer 133, 487–493 (2019).
-
- Furmidge C., Studies at phase interfaces. I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 17, 309–324 (1962).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

