Voltage-energized Calcium-sensitive ATP Production by Mitochondria
- PMID: 31950102
- PMCID: PMC6964030
- DOI: 10.1038/s42255-019-0126-8
Voltage-energized Calcium-sensitive ATP Production by Mitochondria
Erratum in
-
Author Correction: Voltage-energized calcium-sensitive ATP production by mitochondria.Nat Metab. 2019 Nov;1(11):1168. doi: 10.1038/s42255-019-0143-7. Nat Metab. 2019. PMID: 32694862
Abstract
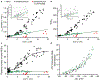
Regulation of ATP production by mitochondria, critical to multicellular life, is poorly understood. Here we investigate the molecular controls of this process in heart and provide a framework for its Ca2+-dependent regulation. We find that the entry of Ca2+ into the matrix through the mitochondrial calcium uniporter (MCU) in heart has neither an apparent cytosolic Ca2+ threshold nor gating function and guides ATP production by its influence on the inner mitochondrial membrane (IMM) potential, ΔΨm. This regulation occurs by matrix Ca2+-dependent modulation of pyruvate and glutamate dehydrogenase activity and not through any effect of Ca2+ on ATP Synthase or on Electron Transport Chain Complexes II, III or IV. Examining the ΔΨm dependence of ATP production over the range of -60 mV to -170 mV in detail reveals that cardiac ATP synthase has a voltage dependence that distinguishes it fundamentally from the previous standard, the bacterial ATP synthase. Cardiac ATP synthase operates with a different ΔΨm threshold for ATP production than bacterial ATP synthase and reveals a concave-upwards shape without saturation. Skeletal muscle MCU Ca2+ flux, while also having no apparent cytosolic Ca2+ threshold, is substantially different from the cardiac MCU, yet the ATP synthase voltage dependence in skeletal muscle is identical to that in the heart. These results suggest that while the conduction of cytosolic Ca2+ signals through the MCU appears to be tissue-dependent, as shown by earlier work1, the control of ATP synthase by ΔΨm appears to be broadly consistent among tissues but is clearly different from bacteria.
Conflict of interest statement
Competing Interests Statement The authors declare no competing financial interests.
Figures

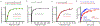
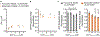






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

