Glucose Effectiveness from Short Insulin-Modified IVGTT and Its Application to the Study of Women with Previous Gestational Diabetes Mellitus
- PMID: 31950770
- PMCID: PMC7188979
- DOI: 10.4093/dmj.2019.0016
Glucose Effectiveness from Short Insulin-Modified IVGTT and Its Application to the Study of Women with Previous Gestational Diabetes Mellitus
Abstract
Background: This study aimed to design a simple surrogate marker (i.e., predictor) of the minimal model glucose effectiveness (SG), namely calculated SG (CSG), from a short insulin-modified intravenous glucose tolerance test (IM-IVGTT), and then to apply it to study women with previous gestational diabetes mellitus (pGDM).
Methods: CSG was designed using the stepwise model selection approach on a population of subjects (n=181) ranging from normal tolerance to type 2 diabetes mellitus (T2DM). CSG was then tested on a population of women with pGDM (n=57). Each subject underwent a 3-hour IM-IVGTT; women with pGDM were observed early postpartum and after a follow-up period of up to 7 years and classified as progressors (PROG) or non-progressors (NONPROG) to T2DM. The minimal model analysis provided a reference SG.
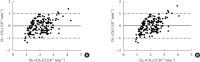
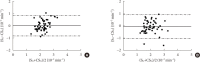
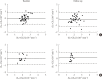
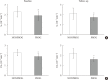
Results: CSG was described as CSG=1.06×10⁻²+5.71×10⁻²×KG/Gpeak, KG being the mean slope (absolute value) of loge glucose in 10-25- and 25-50-minute intervals, and Gpeak being the maximum of the glucose curve. Good agreement between CSG and SG in the general population and in the pGDM group, both at baseline and follow-up (even in PROG and NONPROG subgroups), was shown by the Bland-Altman plots (<5% observations outside limits of agreement), and by the test for equivalence (equivalence margin not higher than one standard deviation). At baseline, the PROG subgroup showed significantly lower SG and CSG values compared to the NONPROG subgroup (P<0.03).
Conclusion: CSG is a valid SG predictor. In the pGDM group, glucose effectiveness appeared to be impaired in women progressing to T2DM.
Keywords: Diabetes mellitus, type 2; Diabetes, gestational; Glucose metabolism disorders; Glucose tolerance test; Models, theoretical.
Copyright © 2020 Korean Diabetes Association.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab. 2001;281:E693–E703. - PubMed
-
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994;43:587–592. - PubMed
-
- Alford FP, Henriksen JE, Rantzau C, Beck-Nielsen H. Glucose effectiveness is a critical pathogenic factor leading to glucose intolerance and type 2 diabetes: an ignored hypothesis. Diabetes Metab Res Rev. 2018;34:e2989. - PubMed
-
- Morettini M, Di Nardo F, Ingrillini L, Fioretti S, Gobl C, Kautzky-Willer A, Tura A, Pacini G, Burattini L. Glucose effectiveness and its components in relation to body mass index. Eur J Clin Invest. 2019:e13099. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

