Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer
- PMID: 31951251
- PMCID: PMC7144521
- DOI: 10.1084/jem.20191869
Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer
Abstract
Experimental and clinical evidence suggests that tumor-associated macrophages (TAMs) play important roles in cancer progression. Here, we have characterized the ontogeny and function of TAM subsets in a mouse model of metastatic ovarian cancer that is representative for visceral peritoneal metastasis. We show that the omentum is a critical premetastatic niche for development of invasive disease in this model and define a unique subset of CD163+ Tim4+ resident omental macrophages responsible for metastatic spread of ovarian cancer cells. Transcriptomic analysis showed that resident CD163+ Tim4+ omental macrophages were phenotypically distinct and maintained their resident identity during tumor growth. Selective depletion of CD163+ Tim4+ macrophages in omentum using genetic and pharmacological tools prevented tumor progression and metastatic spread of disease. These studies describe a specific role for tissue-resident macrophages in the invasive progression of metastatic ovarian cancer. The molecular pathways of cross-talk between tissue-resident macrophages and disseminated cancer cells may represent new targets to prevent metastasis and disease recurrence.
© 2020 Etzerodt et al.
Conflict of interest statement
Disclosures: Dr. Etzerodt reported personal fees from Stipe Therapeutics outside the submitted work. No other disclosures were reported.
Figures

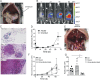


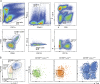









Comment in
-
The omentum, a niche for premetastatic ovarian cancer.J Exp Med. 2020 Apr 6;217(4):e20192312. doi: 10.1084/jem.20192312. J Exp Med. 2020. PMID: 32103261 Free PMC article.
References
-
- Abubaker K., Luwor R.B., Zhu H., McNally O., Quinn M.A., Burns C.J., Thompson E.W., Findlay J.K., and Ahmed N.. 2014. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer. 14:317 10.1186/1471-2407-14-317 - DOI - PMC - PubMed
-
- AlHossiny M., Luo L., Frazier W.R., Steiner N., Gusev Y., Kallakury B., Glasgow E., Creswell K., Madhavan S., Kumar R., and Upadhyay G.. 2016. Ly6E/K signaling to TGFβ promotes breast cancer progression, immune escape, and drug resistance. Cancer Res. 76:3376–3386. 10.1158/0008-5472.CAN-15-2654 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

