A reverse translational study on the effect of rituximab, rituximab plus belimumab, or bortezomib on the humoral autoimmune response in SLE
- PMID: 31951278
- PMCID: PMC7516125
- DOI: 10.1093/rheumatology/kez623
A reverse translational study on the effect of rituximab, rituximab plus belimumab, or bortezomib on the humoral autoimmune response in SLE
Abstract
Objectives: SLE is a severe autoimmune disease characterized by autoreactive B cells and IC formation, which causes systemic inflammation. B cell-targeted therapy could be a promising treatment strategy in SLE patients; nevertheless, randomized clinical trials have not always been successful. However, some groups have demonstrated beneficial effects in severe SLE patients with off-label rituximab (RTX) with belimumab (BLM), or bortezomib (BTZ), which targeted different B cells subsets. This study assembled sera from SLE cohorts treated with RTX+BLM (n = 15), BTZ (n = 11) and RTX (n = 16) to get an in-depth insight into the immunological effects of these therapies on autoantibodies and IC formation.
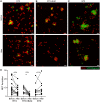
Methods: Autoantibodies relevant for IC formation and the avidity of anti-dsDNA were determined by ELISA. IC-mediated inflammation was studied by complement levels and ex vivo serum-induced neutrophil extracellular trap formation.
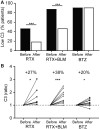
Results: Reductions in autoantibodies were observed after all approaches, but the spectrum differed depending upon the treatment. Specifically, only RTX+BLM significantly decreased anti-C1q. Achieving seronegativity of ≥1 autoantibody, specifically anti-C1q, was associated with lower disease activity. In all SLE patients, the majority of anti-dsDNA autoantibodies had low avidity. RTX+BLM significantly reduced low-, medium- and high-avidity anti-dsDNA, while RTX and BTZ only significantly reduced medium avidity. IC-mediated inflammation, measured by C3 levels and neutrophil extracellular trap formation, improved after RTX+BLM and RTX but less after BTZ.
Conclusion: This study demonstrated the impact of different B cell-targeted strategies on autoantibodies and IC formation and their potential clinical relevance in SLE.
Keywords: B cell–targeted therapies; SLE; autoantibodies; immune-complex formation; neutrophil extracellular traps.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

