Reductive Electrophotocatalysis: Merging Electricity and Light To Achieve Extreme Reduction Potentials
- PMID: 31951390
- PMCID: PMC7023851
- DOI: 10.1021/jacs.9b10678
Reductive Electrophotocatalysis: Merging Electricity and Light To Achieve Extreme Reduction Potentials
Abstract
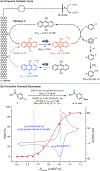
We describe a new electrophotocatalytic strategy that harnesses the power of light and electricity to generate an excited radical anion with a reducing potential of -3.2 V vs SCE, which can be used to activate substrates with very high reduction potentials (Ered ≈ -1.9 to -2.9 V). The resultant aryl radicals can be engaged in various synthetically useful transformations to furnish arylboronate, arylstannane, and biaryl products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Zhang N; Samanta SR; Rosen BM; Percec V Single Electron Transfer in Radical Ion and Radical-Mediated Organic, Materials and Polymer Synthesis. Chem. Rev 2014, 114, 5848–5958. - PubMed
- van der Vlugt JI Radical-Type Reactivity and Catalysis by Single-Electron Transfer to or from Redox-Active Ligands. Chem. -Eur. J 2019, 25, 2651–2662. - PMC - PubMed
- Plesniak MP; Huang H-M; Procter DJ Radical Cascade Reactions Triggered by Single Electron Transfer. Nat. Rev. Chem 2017, 1, 0077.
- Ashby EC Single-Electron Transfer, a Major Reaction Pathway in Organic Chemistry. An Answer to Recent Criticisms. Acc. Chem. Res 1988, 21, 414–421.
- Zhang C; Tang C; Jiao N Recent Advances in Copper-Catalyzed Dehydrogenative Functionalization via a Single Electron Transfer (SET) Process. Chem. Soc. Rev 2012, 41, 3464–3484. - PubMed
- Broggi J; Terme T; Vanelle P Organic Electron Donors as Powerful Single-Electron Reducing Agents in Organic Synthesis. Angew. Chem., Int. Ed 2014, 53, 384–413. - PubMed
- Roth HG; Romero NA; Nicewicz DA Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723.
-
- For representative reviews on visible-light photocatalysis, see: Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035–10074. - PMC - PubMed
- Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075–10166. - PubMed
- Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363. - PMC - PubMed
- Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem 2017, 1, 0052.
- Narayanam JMR; Stephenson CRJ Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev 2011, 40, 102–113. - PubMed
- Fukuzumi S; Ohkubo K Organic synthetic transformations using organic dyes as photoredox catalysts. Org. Biomol. Chem 2014, 12, 6059–6071. - PubMed
-
- For representative reviews on electrochemistry, see: (a)Yan M; Kawamata Y; Baran PS Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev 2017, 117, 13230–13319. - PMC - PubMed
- Sauer GS; Lin S An Electrocatalytic Approach to the Radical Difunctionalization of Alkenes. ACS Catal. 2018, 8, 5175–5187.
- Meyer TH; Finger LH; Gandeepan P; Ackermann L Resource Economy by Metallaelectrocatalysis: Merging Electrochemistry and C−H Activation. Trends in Chem. 2019, 1, 63–76.
- Moeller KD Using Physical Organic Chemistry To Shape the Course of Electrochemical Reactions. Chem. Rev 2018, 118, 4817–4833. - PubMed
- Waldvogel SR; Lips S; Selt M; Riehl B; Kampf CJ Electrochemical Arylation Reaction. Chem. Rev 2018, 118, 6706–6765. - PubMed
- Nutting JE; Rafiee M; Stahl SS Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions. Chem. Rev 2018, 118, 4834–4885. - PMC - PubMed
- Tang S; Liu Y; Lei A Electrochemical Oxidative Cross-coupling with Hydrogen Evolution: A Green and Sustainable Way for Bond Formation. Chem. 2018, 4, 27–45.
-
- Peters BK; Rodriguez KX; Reisberg SH; Beil SB; Hickey DP; Kawamata Y; Collins M; Starr J; Chen L; Udyavara S; Klunder K; Gorey TJ; Anderson SL; Neurock M; Minteer SD; Baran PS Scalable and Safe Synthetic Organic Electroreduction Inspired by Li-Ion Battery Chemistry. Science 2019, 363, 838–845. - PMC - PubMed
- Perkins RJ; Pedro DJ; Hansen EC Electrochemical Nickel Catalysis for Sp2-Sp3 Cross-Electrophile Coupling Reactions of Unactivated Alkyl Halides. Org. Lett 2017, 19, 3755–3758. - PubMed
- Sun G; Ren S; Zhu X; Huang M; Wan Y Direct Arylation of Pyrroles via Indirect Electroreductive C−H Functionalization Using Perylene Bisimide as an Electron-Transfer Mediator. Org. Lett 2016, 18, 544–547. - PubMed
- Edinger C; Waldvogel SR Electrochemical Deoxygenation of Aromatic Amides and Sulfoxides. Eur. J. Org. Chem 2014, 2014, 5144–5148.
-
- Recent examples of combining electrochemistry and photochemistry for organic synthesis: (a)Wang F; Stahl SS Merging Photochemistry with Electrochemistry: Functional-Group Tolerant Electrochemical Amination of C(sp3)−H Bonds. Angew. Chem., Int. Ed 2019, 58, 6385–6390. - PMC - PubMed
- Zhang L; Liardet L; Luo J; Ren D; Gratzel M; Hu X Photoelectrocatalytic Arene C−H Amination. Nat. Catal 2019, 2, 366–373. - PMC - PubMed
- Yan H; Hou Z-W; Xu H-C Photoelectrochemical C−H Alkylation of Heteroarenes with Organo-trifluoroborates. Angew. Chem., Int. Ed 2019, 58, 4592–4595. - PubMed
- Zhang W; Carpenter K; Lin S Electrochemistry Broadens the Scope of Flavin Photocatalysis: Photoelectrocatalytic Oxidation of Unactivated Alcohols. Angew. Chem., Int. Ed 2020, 59, 409–417. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

