Casticin Induces DNA Damage and Affects DNA Repair Associated Protein Expression in Human Lung Cancer A549 Cells (Running Title: Casticin Induces DNA Damage in Lung Cancer Cells)
- PMID: 31952105
- PMCID: PMC7024307
- DOI: 10.3390/molecules25020341
Casticin Induces DNA Damage and Affects DNA Repair Associated Protein Expression in Human Lung Cancer A549 Cells (Running Title: Casticin Induces DNA Damage in Lung Cancer Cells)
Erratum in
-
Erratum: Cheng, Z.Y., et al. Casticin Induces DNA Damage and Affects DNA Repair Associated Protein Expression in Human Lung Cancer A549 Cells (Running Title: Casticin Induces DNA Damage in Lung Cancer Cells). Molecules 2020, 25, 341.Molecules. 2021 Jan 8;26(2):292. doi: 10.3390/molecules26020292. Molecules. 2021. PMID: 33430168 Free PMC article.
Abstract
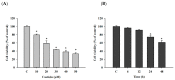
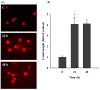
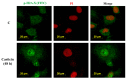
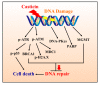
Casticin was obtained from natural plants, and it has been shown to exert biological functions; however, no report concerns the induction of DNA damage and repair in human lung cancer cells. The objective of this study was to investigate the effects and molecular mechanism of casticin on DNA damage and repair in human lung cancer A549 cells. Cell viability was determined by flow cytometric assay. The DNA damage was evaluated by 4',6-diamidino-2-phenylindole (DAPI) staining and electrophoresis which included comet assay and DNA gel electrophoresis. The protein levels associated with DNA damage and repair were analyzed by western blotting. The expression and translocation of p-H2A.X were observed by confocal laser microscopy. Casticin reduced total viable cell number and induced DNA condensation, fragmentation, and damage in A549 cells. Furthermore, casticin increased p-ATM at 6 h and increased p-ATR and BRCA1 at 6-24 h treatment but decreased p-ATM at 24-48 h, as well as decreased p-ATR and BRCA1 at 48 h. Furthermore, casticin decreased p-p53 at 6-24 h but increased at 48 h. Casticin increased p-H2A.X and MDC1 at 6-48 h treatment. In addition, casticin increased PARP (cleavage) at 6, 24, and 48 h treatment, DNA-PKcs and MGMT at 48 h in A549 cells. Casticin induced the expressions and nuclear translocation of p-H2AX in A549 cells by confocal laser microscopy. Casticin reduced cell number through DNA damage and condensation in human lung cancer A549 cells.
Keywords: DNA condensation and repair; DNA damage; casticin; human lung cancer A549 cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Casticin Induces DNA Damage and Impairs DNA Repair in Human Bladder Cancer TSGH-8301 Cells.Anticancer Res. 2019 Apr;39(4):1839-1847. doi: 10.21873/anticanres.13291. Anticancer Res. 2019. PMID: 30952724
-
Casticin induces DNA damage and inhibits DNA repair-associated protein expression in B16F10 mouse melanoma cancer cells.Oncol Rep. 2016 Oct;36(4):2094-100. doi: 10.3892/or.2016.5027. Epub 2016 Aug 17. Oncol Rep. 2016. PMID: 27572101
-
Gallic acid induces DNA damage and inhibits DNA repair-associated protein expression in human oral cancer SCC-4 cells.Anticancer Res. 2015 Apr;35(4):2077-84. Anticancer Res. 2015. PMID: 25862863
-
Kaempferol induces DNA damage and inhibits DNA repair associated protein expressions in human promyelocytic leukemia HL-60 cells.Am J Chin Med. 2015;43(2):365-82. doi: 10.1142/S0192415X1550024X. Epub 2015 Mar 17. Am J Chin Med. 2015. PMID: 25779644
-
Ouabain Induces DNA Damage in Human Osteosarcoma U-2 OS Cells and Alters the Expression of DNA Damage and DNA Repair-associated Proteins.In Vivo. 2021 Sep-Oct;35(5):2687-2696. doi: 10.21873/invivo.12552. In Vivo. 2021. PMID: 34410957 Free PMC article.
Cited by
-
Casticin protected against neuronal injury and inhibited the TLR4/NF-κB pathway after middle cerebral artery occlusion in rats.Pharmacol Res Perspect. 2021 Apr;9(2):e00752. doi: 10.1002/prp2.752. Pharmacol Res Perspect. 2021. PMID: 33704926 Free PMC article.
-
Deciphering the effects of bixin on pulmonary alveolar adenocarcinoma migration and proliferation via targeting BAX/BCL-2 and Cyclin D1.Sci Rep. 2025 Apr 29;15(1):15109. doi: 10.1038/s41598-025-96788-9. Sci Rep. 2025. PMID: 40301461 Free PMC article.
-
An Overview of the Potential Antineoplastic Effects of Casticin.Molecules. 2020 Mar 12;25(6):1287. doi: 10.3390/molecules25061287. Molecules. 2020. PMID: 32178324 Free PMC article. Review.
-
Casticin Attenuates Stemness in Cervical Cancer Stem-Like Cells by Regulating Activity and Expression of DNMT1.Chin J Integr Med. 2023 Mar;29(3):224-232. doi: 10.1007/s11655-022-3469-z. Epub 2022 Jul 9. Chin J Integr Med. 2023. PMID: 35809177
-
Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer.Biomedicines. 2021 Jun 18;9(6):689. doi: 10.3390/biomedicines9060689. Biomedicines. 2021. PMID: 34207313 Free PMC article. Review.
References
-
- Roointan A., Sharifi-Rad M., Badrzadeh F., Sharifi-Rad J. A comparison between PLGA-PEG and NIPAAm-MAA nanocarriers in curcumin delivery for hTERT silencing in lung cancer cell line. Cell. Mol. Boil. 2016;62:51–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

