Grafting of Diazonium Salts on Surfaces: Application to Biosensors
- PMID: 31952195
- PMCID: PMC7168266
- DOI: 10.3390/bios10010004
Grafting of Diazonium Salts on Surfaces: Application to Biosensors
Abstract
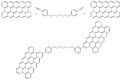
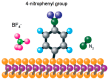
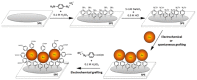
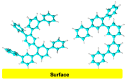
This review is divided into two parts; the first one summarizes the main features of surface modification by diazonium salts with a focus on most recent advances, while the second part deals with diazonium-based biosensors including small molecules of biological interest, proteins, and nucleic acids.
Keywords: biosensor; diazonium; surface modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




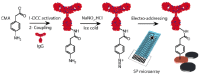
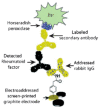

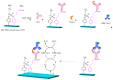

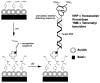
References
-
- Allongue P., Delamar M., Desbat B., Fagebaume O., Hitmi R., Pinson J., Savéant J.-M. Covalent Modification of Carbon Surfaces by Aryl Radicals Generated from the Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1997;119:201–207. doi: 10.1021/ja963354s. - DOI
-
- Berisha A., Chehimi M.M., Pinson J., Podvorica F.I. Electrode surface modification using diazonium salts. Electroanal. Chem. 2016;26:115–225.
-
- Aryl Diazonium Salts . In: New Coupling Agents in Polymer and Surface Science. Chehimi M.M., editor. Wiley-VCH; Weinheim, Germany: 2012.
-
- Jiang C., Moraes Silva S., Fan S., Wu Y., Alam M.T., Liu G., Gooding J.J. Aryldiazonium salt derived mixed organic layers: From surface chemistry to their applications. J. Electroanal. Chem. 2017;785:265–278. doi: 10.1016/j.jelechem.2016.11.043. - DOI
-
- Assresahegn B.D., Brousse T., Belanger D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon. 2015;92:362–381. doi: 10.1016/j.carbon.2015.05.030. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

