The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: the DOLPHIN trial protocol of a multi-centre randomised controlled trial
- PMID: 31952493
- PMCID: PMC6969462
- DOI: 10.1186/s12879-020-4781-x
The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: the DOLPHIN trial protocol of a multi-centre randomised controlled trial
Abstract
Background: Critically ill patients undergo extensive physiological alterations that will have impact on antibiotic pharmacokinetics. Up to 60% of intensive care unit (ICU) patients meet the pharmacodynamic targets of beta-lactam antibiotics, with only 30% in fluoroquinolones. Not reaching these targets might increase the chance of therapeutic failure, resulting in increased mortality and morbidity, and antibiotic resistance. The DOLPHIN trial was designed to demonstrate the added value of therapeutic drug monitoring (TDM) of beta-lactam and fluoroquinolones in critically ill patients in the ICU.
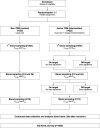
Methods: A multi-centre, randomised controlled trial (RCT) was designed to assess the efficacy and cost-effectiveness of model-based TDM of beta-lactam and fluoroquinolones. Four hundred fifty patients will be included within 24 months after start of inclusion. Eligible patients will be randomly allocated to either study group: the intervention group (active TDM) or the control group (non-TDM). In the intervention group dose adjustment of the study antibiotics (cefotaxime, ceftazidime, ceftriaxone, cefuroxime, amoxicillin, amoxicillin with clavulanic acid, flucloxacillin, piperacillin with tazobactam, meropenem, and ciprofloxacin) on day 1, 3, and 5 is performed based upon TDM with a Bayesian model. The primary outcome will be ICU length of stay. Other outcomes amongst all survival, disease severity, safety, quality of life after ICU discharge, and cost effectiveness will be included.
Discussion: No trial has investigated the effect of early TDM of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients. The findings from the DOLPHIN trial will possibly lead to new insights in clinical management of critically ill patients receiving antibiotics. In short, to TDM or not to TDM?
Trial registration: EudraCT number: 2017-004677-14. Sponsor protocol name: DOLPHIN. Registered 6 March 2018 . Protocol Version 6, Protocol date: 27 November 2019.
Keywords: Antibiotic; Beta-lactam; Critically ill patients; Fluoroquinolones; Length of stay; Pharmacodynamics; Pharmacokinetics; Randomised controlled trial; Therapeutic drug monitoring.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Shorr AF. An update on cost-effectiveness analysis in critical care. Curr Opin Crit Care. 2002;8(4):337–343. - PubMed
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. Jama. 2009;302(21):2323–2329. - PubMed
-
- SepNet Critical Care Trials G Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42(12):1980–1989. - PubMed
-
- Esteban A, Frutos-Vivar F, Ferguson ND, Penuelas O, Lorente JA, Gordo F, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

