Effects of probiotics on type II diabetes mellitus: a meta-analysis
- PMID: 31952517
- PMCID: PMC6966830
- DOI: 10.1186/s12967-020-02213-2
Effects of probiotics on type II diabetes mellitus: a meta-analysis
Erratum in
-
Correction to: Effects of probiotics on type II diabetes mellitus: a meta-analysis.J Transl Med. 2020 Feb 28;18(1):105. doi: 10.1186/s12967-020-02274-3. J Transl Med. 2020. PMID: 32111256 Free PMC article.
Abstract
Objective: The purpose of the present study was to evaluate the effectiveness of probiotics on type II diabetes mellitus (T2DM).
Methods: We performed a comprehensive search on PubMed, Web of Science, China National Knowledge Infrastructure, Chinese Scientific Journal Databases, Wan Fang database and China biology medicine disc for relevant studies published before June 2019. Glycated hemoglobin A1c (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR) and fasting blood glucose (FBG) were used as indicators for T2DM. Inverse-variance weighted mean difference (WMD) with 95% confidence interval (CI) was calculated for the mean HbA1c, FBG and HOMA-IR changes from baseline.
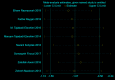
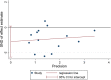
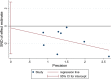
Results: 15 randomized controlled trials (RCT) with a total of 902 participants were included into the meta-analysis. Considering the clinical heterogeneity caused by variation of dosage and duration of probiotic treatment, random-effects model was used to estimate the pooled WMD. Significantly greater reduction in HbA1c% (WMD = - 0.24, 95% CI [- 0.44, - 0.04], p = 0.02), FBG (WMD = - 0.44 mmol/L, 95% CI [- 0.74, - 0.15], p = 0.003) and HOMA-IR (WMD = - 1.07, 95% CI [- 1.58, - 0.56], p < 0.00001) were observed in probiotics treated group. Further sensitivity analysis verified the reliability and stability of our results.
Conclusion: The results of our meta-analysis indicated that probiotics treatment may reduce HbA1c, FBG and insulin resistance level in T2DM patients. More clinical data and research into the mechanism of probiotics are needed to clarify the role of probiotics in T2DM.
Keywords: Gut microbiota; Meta-analysis; Probiotic; Type II diabetes mellitus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






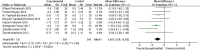

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

