Evolving Significance of Tumor-Normal Sequencing in Cancer Care
- PMID: 31952779
- PMCID: PMC8923150
- DOI: 10.1016/j.trecan.2019.11.006
Evolving Significance of Tumor-Normal Sequencing in Cancer Care
Abstract
Molecular tests assist at various stages of cancer patient management, including providing diagnosis, predicting prognosis, identifying therapeutic targets, and determining hereditary cancer risk. The current testing paradigm involves germline testing in a subset of patients determined to be at high risk for having a hereditary cancer syndrome, and tumor-only sequencing for treatment decisions in advanced cancer patients. A major limitation of tumor-only sequencing is its inability to distinguish germline versus somatic mutations. Tumor-normal sequencing has emerged as a comprehensive analysis for both hereditary cancer predisposition and somatic profiling. Here, we review recent studies involving tumor-normal sequencing, discuss its benefits in clinical care, challenges for its implementation, and novel insights it has provided regarding tumor biology and germline contribution to cancer.
Keywords: hereditary cancer predisposition; tumor sequencing; tumor-normal sequencing.
Copyright © 2019 Elsevier Inc. All rights reserved.
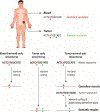
Figures


References
-
- Garber JE and Offit K (2005) Hereditary cancer predisposition syndromes. J Clin Oncol 23, 276–292 - PubMed
-
- Lichtenstein P, et al. (2000) Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343, 78–85 - PubMed
-
- Robson ME, et al. (2015) American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33, 3660–3667 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

