Mutant p53 on the Path to Metastasis
- PMID: 31952783
- PMCID: PMC7485681
- DOI: 10.1016/j.trecan.2019.11.004
Mutant p53 on the Path to Metastasis
Abstract
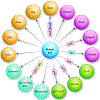
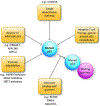
Metastasis contributes to the vast majority of cancer-related mortality. Regulatory mechanisms of the multistep invasion-metastasis cascade are being unraveled. TP53 is the most frequently mutated gene across human cancers. Accumulating evidence has shown that mutations of TP53 not only lead to loss of function or dominant negative effects, but also promotes a gain of function. Specifically, gain of function mutant p53 promotes cancer cell motility, invasion, and metastasis. Here, we summarize the mechanisms and functions of mutant p53 that foster metastasis in different types of cancers. We also discuss the prognostic value of mutant p53 and current status of therapeutic strategies targeting mutant p53. Future studies will shed light on discovering novel mechanisms of mutant p53-driven cancer metastasis and developing innovative therapeutics to improve clinical outcomes in patients harboring p53 mutations.
Keywords: epithelial-to-mesenchymal transition (EMT); extracellular matrix (ECM); metastasis; mutant p53; receptor tyrosine kinase (RTK); therapeutics.
Published by Elsevier Inc.
Figures
References
-
- Powell E et al. (2014) Contribution of p53 to metastasis. Cancer Discov. 4, 405–414. DOI: 10.1158/2159-8290.CD-13-0136 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

