Synaptotagmin-1 and Doc2b Exhibit Distinct Membrane-Remodeling Mechanisms
- PMID: 31952804
- PMCID: PMC7002981
- DOI: 10.1016/j.bpj.2019.12.021
Synaptotagmin-1 and Doc2b Exhibit Distinct Membrane-Remodeling Mechanisms
Abstract
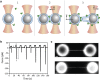
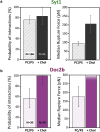
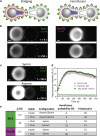
Synaptotagmin-1 (Syt1) is a calcium sensor protein that is critical for neurotransmission and is therefore extensively studied. Here, we use pairs of optically trapped beads coated with SNARE-free synthetic membranes to investigate Syt1-induced membrane remodeling. This activity is compared with that of Doc2b, which contains a conserved C2AB domain and induces membrane tethering and hemifusion in this cell-free model. We find that the soluble C2AB domain of Syt1 strongly affects the probability and strength of membrane-membrane interactions in a strictly Ca2+- and protein-dependent manner. Single-membrane loading of Syt1 yielded the highest probability and force of membrane interactions, whereas in contrast, Doc2b was more effective after loading both membranes. A lipid-mixing assay with confocal imaging reveals that both Syt1 and Doc2b are able to induce hemifusion; however, significantly higher Syt1 concentrations are required. Consistently, both C2AB fragments cause a reduction in the membrane-bending modulus, as measured by a method based on atomic force microscopy. This lowering of the energy required for membrane deformation may contribute to Ca2+-induced fusion.
Copyright © 2019 Biophysical Society. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

