Immune - Goblet cell interaction in the conjunctiva
- PMID: 31953222
- PMCID: PMC7125021
- DOI: 10.1016/j.jtos.2019.12.006
Immune - Goblet cell interaction in the conjunctiva
Abstract
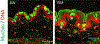
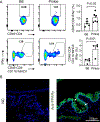
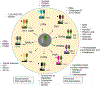
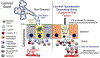
The conjunctiva is a goblet cell rich mucosal tissue. Goblet cells are supported by tear growth factors and IL-13 produced by resident immune cells. Goblet cell secretions are essential for maintaining tear stability and ocular surface homeostasis. In addition to producing tear stabilizing mucins, they also produce cytokines and retinoic acid that condition monocyte-derived phagocytic cells in the conjunctiva. Aqueous tear deficiency from lacrimal gland disease and systemic inflammatory conditions results in goblet cell loss that amplifies dry eye severity. Reduced goblet cell density is correlated with more severe conjunctival disease, increased IFN-γ expression and antigen presenting cell maturation. Sterile Alpha Motif (SAM) pointed domain epithelial specific transcription factor (Spdef) gene deficient mice that lack goblet cells have increased infiltration of monocytes and dendritic cells with greater IL-12 expression in the conjunctiva. Similar findings were observed in the conjunctiva of aged mice. Reduced retinoic acid receptor (RXRα) signaling also increases conjunctival monocyte infiltration, IFN-γ expression and goblet cell loss. Evidence suggests that dry eye therapies that suppress IFN-γ expression preserve conjunctival goblet cell number and function and should be considered in aqueous deficiency.
Keywords: Conjunctiva; Goblet cell; Immune response; Immunoregulation; Interferon gamma; Retinoic acid; Retinoid receptor.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest None of the authors have any financial or personal relationships to disclose that would cause a conflict of interest regarding this article.
Figures


References
-
- Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. American journal of ophthalmology. 2017;182:90–98. - PubMed
-
- Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res 2002;21(6):555–576. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

