Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma
- PMID: 31953246
- PMCID: PMC10765595
- DOI: 10.1158/2326-6066.CIR-19-0293
Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma
Abstract
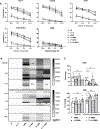
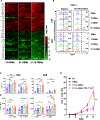
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death in the world, and curative systemic therapies are lacking. Chimeric antigen receptor (CAR)-expressing T cells induce robust antitumor responses in patients with hematologic malignancies but have limited efficacy in patients with solid tumors, including HCC. IL15 and IL21 promote T-cell expansion, survival, and function and can improve the antitumor properties of T cells. We explored whether transgenic expression of IL15 and/or IL21 enhanced glypican-3-CAR (GPC3-CAR) T cells' antitumor properties against HCC. We previously optimized the costimulation in GPC3-CARs and selected a second-generation GPC3-CAR incorporating a 4-1BB costimulatory endodomain (GBBz) for development. Here, we generated constructs encoding IL15, IL21, or both with GBBz (15.GBBz, 21.GBBz, and 21.15.GBBz, respectively) and examined the ability of transduced T cells to kill, produce effector cytokines, and expand in an antigen-dependent manner. We performed gene-expression and phenotypic analyses of GPC3-CAR T cells and CRISPR-Cas9 knockout of the TCF7 gene. Finally, we measured GPC3-CAR T-cell antitumor activity in murine xenograft models of GPC3+ tumors. The increased proliferation of 21.15.GBBz T cells was at least in part dependent on the upregulation and maintenance of TCF-1 (encoded by TCF7) and associated with a higher percentage of stem cell memory and central memory populations after manufacturing. T cells expressing 21.15.GBBz had superior in vitro and in vivo expansion and persistence, and the most robust antitumor activity in vivo These results provided preclinical evidence to support the clinical evaluation of 21.15.GPC3-CAR T cells in patients with HCC.
©2020 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest disclosure statement:
SAB, PR, LSM and AH have pending patent applications covering GPC3-CARs. Other co-authors report no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

