Protein secondary structure determines the temporal relationship between folding and disulfide formation
- PMID: 31953323
- PMCID: PMC7039548
- DOI: 10.1074/jbc.RA119.011983
Protein secondary structure determines the temporal relationship between folding and disulfide formation
Abstract
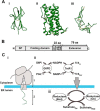
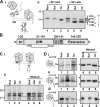
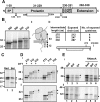
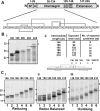
How and when disulfide bonds form in proteins relative to the stage of their folding is a fundamental question in cell biology. Two models describe this relationship: the folded precursor model, in which a nascent structure forms before disulfides do, and the quasi-stochastic model, where disulfides form prior to folding. Here we investigated oxidative folding of three structurally diverse substrates, β2-microglobulin, prolactin, and the disintegrin domain of ADAM metallopeptidase domain 10 (ADAM10), to understand how these mechanisms apply in a cellular context. We used a eukaryotic cell-free translation system in which we could identify disulfide isomers in stalled translation intermediates to characterize the timing of disulfide formation relative to translocation into the endoplasmic reticulum and the presence of non-native disulfides. Our results indicate that in a domain lacking secondary structure, disulfides form before conformational folding through a process prone to nonnative disulfide formation, whereas in proteins with defined secondary structure, native disulfide formation occurs after partial folding. These findings reveal that the nascent protein structure promotes correct disulfide formation during cotranslational folding.
Keywords: disulfide; endoplasmic reticulum (ER); folded precursor model; nascent chain; protein disulfide isomerase; protein folding; protein homeostasis; protein secretion; protein structure; protein translocation; quasi-stochastic model.
© 2020 Robinson et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

