The small molecule GAT1508 activates brain-specific GIRK1/2 channel heteromers and facilitates conditioned fear extinction in rodents
- PMID: 31953327
- PMCID: PMC7076198
- DOI: 10.1074/jbc.RA119.011527
The small molecule GAT1508 activates brain-specific GIRK1/2 channel heteromers and facilitates conditioned fear extinction in rodents
Abstract
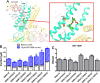
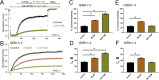
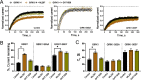
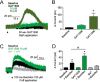
G-protein-gated inwardly-rectifying K+ (GIRK) channels are targets of Gi/o-protein-signaling systems that inhibit cell excitability. GIRK channels exist as homotetramers (GIRK2 and GIRK4) or heterotetramers with nonfunctional homomeric subunits (GIRK1 and GIRK3). Although they have been implicated in multiple conditions, the lack of selective GIRK drugs that discriminate among the different GIRK channel subtypes has hampered investigations into their precise physiological relevance and therapeutic potential. Here, we report on a highly-specific, potent, and efficacious activator of brain GIRK1/2 channels. Using a chemical screen and electrophysiological assays, we found that this activator, the bromothiophene-substituted small molecule GAT1508, is specific for brain-expressed GIRK1/2 channels rather than for cardiac GIRK1/4 channels. Computational models predicted a GAT1508-binding site validated by experimental mutagenesis experiments, providing insights into how urea-based compounds engage distant GIRK1 residues required for channel activation. Furthermore, we provide computational and experimental evidence that GAT1508 is an allosteric modulator of channel-phosphatidylinositol 4,5-bisphosphate interactions. Through brain-slice electrophysiology, we show that subthreshold GAT1508 concentrations directly stimulate GIRK currents in the basolateral amygdala (BLA) and potentiate baclofen-induced currents. Of note, GAT1508 effectively extinguished conditioned fear in rodents and lacked cardiac and behavioral side effects, suggesting its potential for use in pharmacotherapy for post-traumatic stress disorder. In summary, our findings indicate that the small molecule GAT1508 has high specificity for brain GIRK1/2 channel subunits, directly or allosterically activates GIRK1/2 channels in the BLA, and facilitates fear extinction in a rodent model.
Keywords: GIRK channels; PIP2; basolateral amygdala; medicinal chemistry; neurophysiology; phosphoinositide; potassium channel; small molecule; specific activator.
© 2020 Xu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

