Vaporized Cannabis Extracts Have Reinforcing Properties and Support Conditioned Drug-Seeking Behavior in Rats
- PMID: 31953372
- PMCID: PMC7046447
- DOI: 10.1523/JNEUROSCI.2416-19.2020
Vaporized Cannabis Extracts Have Reinforcing Properties and Support Conditioned Drug-Seeking Behavior in Rats
Abstract
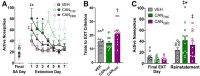
Recent trends in cannabis legalization have increased the necessity to better understand the effects of cannabis use. Animal models involving traditional cannabinoid self-administration approaches have been notoriously difficult to establish and differences in the drug used and its route of administration have limited the translational value of preclinical studies. To address this challenge in the field, we have developed a novel method of cannabis self-administration using response-contingent delivery of vaporized Δ9-tetrahydrocannabinol-rich (CANTHC) or cannabidiol-rich (CANCBD) whole-plant cannabis extracts. Male Sprague-Dawley rats were trained to nose-poke for discrete puffs of CANTHC, CANCBD, or vehicle (VEH) in daily 1 h sessions. Cannabis vapor reinforcement resulted in strong discrimination between active and inactive operanda. CANTHC maintained higher response rates under fixed ratio schedules and higher break points under progressive ratio schedules compared with CANCBD or VEH, and the number of vapor deliveries positively correlated with plasma THC concentrations. Moreover, metabolic phenotyping studies revealed alterations in locomotor activity, energy expenditure, and daily food intake that are consistent with effects in human cannabis users. Furthermore, both cannabis regimens produced ecologically relevant brain concentrations of THC and CBD and CANTHC administration decreased hippocampal CB1 receptor binding. Removal of CANTHC reinforcement (but not CANCBD) resulted in a robust extinction burst and an increase in cue-induced cannabis-seeking behavior relative to VEH. These data indicate that volitional exposure to THC-rich cannabis vapor has bona fide reinforcing properties and collectively support the utility of the vapor self-administration model for the preclinical assessment of volitional cannabis intake and cannabis-seeking behaviors.SIGNIFICANCE STATEMENT The evolving legal landscape concerning recreational cannabis use has increased urgency to better understand its effects on the brain and behavior. Animal models are advantageous in this respect; however, current approaches typically used forced injections of synthetic cannabinoids or isolated cannabis constituents that may not capture the complex effects of volitional cannabis consumption. We have developed a novel model of cannabis self-administration using response-contingent delivery of vaporized cannabis extracts containing high concentrations of Δ9 tetrahydrocannabinol (THC) or cannabidiol. Our data indicate that THC-rich cannabis vapor has reinforcing properties that support stable rates of responding and conditioned drug-seeking behavior. This approach will be valuable for interrogating effects of cannabis and delineating neural mechanisms that give rise to aberrant cannabis-seeking behavior.
Keywords: cannabinoid; cannabis; rat; self-administration; translational; vapor.
Copyright © 2020 the authors.
Figures





Comment in
-
Cannabis Extract Composition Determines Reinforcement in a Vapor Self-Administration Paradigm.J Neurosci. 2020 Aug 12;40(33):6264-6266. doi: 10.1523/JNEUROSCI.0814-20.2020. J Neurosci. 2020. PMID: 32801126 Free PMC article. No abstract available.
References
-
- Berger AL, Henricks AM, Lugo JM, Wright HR, Warrick CR, Sticht MA, Morena M, Bonilla I, Laredo SA, Craft RM, Parsons LH, Grandes PR, Hillard CJ, Hill MN, McLaughlin RJ (2018) The lateral habenula directs coping styles under conditions of stress via recruitment of the endocannabinoid system. Biol Psychiatry 84:611–623. 10.1016/j.biopsych.2018.04.018 - DOI - PMC - PubMed
-
- Carbuto M, Sewell RA, Williams A, Forselius-Bielen K, Braley G, Elander J, Pittman B, Schnakenberg A, Bhakta S, Perry E, Ranganathan M, D'Souza DC, Yale THC Study Group (2012) The safety of studies with intravenous Δ9-tetrahydrocannabinol in humans, with case histories. Psychopharmacology 219:885–896. 10.1007/s00213-011-2417-y - DOI - PubMed
-
- Cook SA, Lowe JA, Martin BR (1998) CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 285:1150–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
