Polypeptide templating for designer hierarchical materials
- PMID: 31953407
- PMCID: PMC6969164
- DOI: 10.1038/s41467-019-14257-0
Polypeptide templating for designer hierarchical materials
Abstract
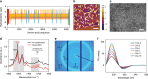
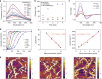
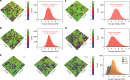
Despite advances in directing the assembly of biomacromolecules into well-defined nanostructures, leveraging pathway complexity of molecular disorder to order transition while bridging materials fabrication from nano- to macroscale remains a challenge. Here, we present templated crystallization of structural proteins to nanofabricate hierarchically structured materials up to centimeter scale, using silk fibroin as an example. The process involves the use of ordered peptide supramolecular assemblies as templates to direct the folding and assembly of silk fibroin into nanofibrillar structures. Silk polymorphs can be engineered by varying the peptide seeds used. Modulation of the relative concentration between silk fibroin and peptide seeds, silk fibroin molecular weight and pH allows control over nanofibrils morphologies and mechanical properties. Finally, facile integration of the bottom-up templated crystallization with emerging top-down techniques enables the generation of macroscopic nanostructured materials with potential applications in information storage/encryption, surface functionalization, and printable three-dimensional constructs of customized architecture and controlled anisotropy.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dunlop JWC, Fratzl P. Biological composites. Annu. Rev. Mater. Res. 2010;40:1–24. doi: 10.1146/annurev-matsci-070909-104421. - DOI
-
- Tagliazucchi M, Szleifer I. Transport mechanisms in nanopores and nanochannels: can we mimic nature? Mater. Today. 2015;18:131–142. doi: 10.1016/j.mattod.2014.10.020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

