Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons
- PMID: 31953414
- PMCID: PMC6969162
- DOI: 10.1038/s41598-019-56930-w
Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons
Abstract
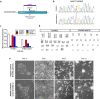
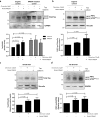
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder characterized by the presence of intracellular aggregates of tau protein and neuronal loss leading to cognitive and motor impairment. Occurrence is mostly sporadic, but rare family clusters have been described. Although the etiopathology of PSP is unknown, mutations in the MAPT/tau gene and exposure to environmental toxins can increase the risk of PSP. Here, we used cell models to investigate the potential neurotoxic effects of heavy metals enriched in a highly industrialized region in France with a cluster of sporadic PSP cases. We found that iPSC-derived iNeurons from a MAPT mutation carrier tend to be more sensitive to cell death induced by chromium (Cr) and nickel (Ni) exposure than an isogenic control line. We hypothesize that genetic variations may predispose to neurodegeneration induced by those heavy metals. Furthermore, using an SH-SY5Y neuroblastoma cell line, we showed that both heavy metals induce cell death by an apoptotic mechanism. Interestingly, Cr and Ni treatments increased total and phosphorylated tau levels in both cell types, implicating Cr and Ni exposure in tau pathology. Overall, this study suggests that chromium and nickel could contribute to the pathophysiology of tauopathies such as PSP by promoting tau accumulation and neuronal cell death.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

