Endothelial Autophagy: an Effective Target for Radiation-induced Cerebral Capillary Damage
- PMID: 31953486
- PMCID: PMC6968992
- DOI: 10.1038/s41598-019-57234-9
Endothelial Autophagy: an Effective Target for Radiation-induced Cerebral Capillary Damage
Abstract
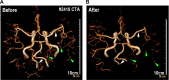
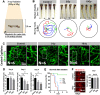
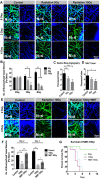
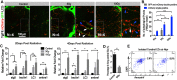
Toxicity to central nervous system tissues is the common side effects for radiotherapy of brain tumor. The radiation toxicity has been thought to be related to the damage of cerebral endothelium. However, because of lacking a suitable high-resolution vivo model, cellular response of cerebral capillaries to radiation remained unclear. Here, we present the flk:eGFP transgenic zebrafish larvae as a feasible model to study the radiation toxicity to cerebral capillary. We showed that, in living zebrafish larvae, radiation could induce acute cerebral capillary shrinkage and blood-flow obstruction, resulting brain hypoxia and glycolysis retardant. Although in vivo neuron damage was also observed after the radiation exposure, further investigation found that they didn't response to the same dosage of radiation in vitro, indicating that radiation induced neuron damage was a secondary-effect of cerebral vascular function damage. In addition, transgenic labeling and qPCR results showed that the radiation-induced acute cerebral endothelial damage was correlated with intensive endothelial autophagy. Different autophagy inhibitors could significantly alleviate the radiation-induced cerebral capillary damage and prolong the survival of zebrafish larvae. Therefore, we showed that radiation could directly damage cerebral capillary, resulting to blood flow deficiency and neuron death, which suggested endothelial autophagy as a potential target for radiation-induced brain toxicity.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

