Diroximel Fumarate Demonstrates an Improved Gastrointestinal Tolerability Profile Compared with Dimethyl Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III EVOLVE-MS-2 Study
- PMID: 31953790
- PMCID: PMC7018784
- DOI: 10.1007/s40263-020-00700-0
Diroximel Fumarate Demonstrates an Improved Gastrointestinal Tolerability Profile Compared with Dimethyl Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III EVOLVE-MS-2 Study
Abstract
Background: Diroximel fumarate (DRF) is a novel oral fumarate approved in the USA for relapsing forms of multiple sclerosis. DRF is converted to monomethyl fumarate, the pharmacologically active metabolite of dimethyl fumarate (DMF). DRF 462 mg and DMF 240 mg produce bioequivalent exposure of monomethyl fumarate and are therefore expected to have similar efficacy/safety profiles; the distinct chemical structure of DRF may contribute to its tolerability profile.
Objectives: The objective of this study was to compare the gastrointestinal tolerability of DRF and DMF over 5 weeks in patients with relapsing-remitting multiple sclerosis.
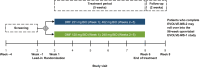
Methods: EVOLVE-MS-2 was a phase III, randomized, double-blind, head-to-head, 5-week study evaluating the gastrointestinal tolerability of DRF 462 mg vs DMF 240 mg, administered twice daily in patients with relapsing-remitting multiple sclerosis, using two self-administered gastrointestinal symptom scales: Individual Gastrointestinal Symptom and Impact Scale (IGISIS) and Global Gastrointestinal Symptom and Impact Scale (GGISIS). The primary endpoint was the number of days with an IGISIS intensity score ≥ 2 relative to exposure. Other endpoints included the degree of gastrointestinal symptom severity measured by IGISIS/GGISIS and assessment of safety/tolerability.
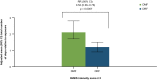
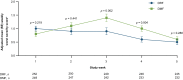
Results: DRF-treated patients experienced a statistically significant reduction (46%) in the number of days with an IGISIS symptom intensity score ≥ 2 compared with DMF-treated patients (rate ratio [95% confidence interval]: 0.54 [0.39-0.75]; p = 0.0003). Lower rates of gastrointestinal adverse events (including diarrhea, nausea, vomiting, and abdominal pain) were observed with DRF than DMF (34.8% vs 49.0%). Fewer patients discontinued DRF than DMF because of adverse events (1.6% vs 5.6%) and gastrointestinal adverse events (0.8% vs 4.8%).
Conclusions: DRF demonstrated an improved gastrointestinal tolerability profile compared with DMF, with less severe gastrointestinal events and fewer days of self-assessed gastrointestinal symptoms, fewer gastrointestinal adverse events, and lower discontinuation rates because of gastrointestinal adverse events.
Clinical trials registration: ClinicalTrials.gov (NCT03093324).
Conflict of interest statement
Robert T. Naismith received compensation as an advisor, consultant, or speaker for Alexion, Alkermes, Biogen, Celgene, EMD Serono, Genentech, Novartis, Sanofi Genzyme, TG Therapeutics, and Viela Bio. Annette Wundes received research support from AbbVie, Alkermes, and Biogen, and compensation as an advisor from Biogen. Tiaf Ziemssen received fees for participation in scientific advisory boards from Bayer, Biogen Idec, Sanofi Genzyme, Merck Serono, Novartis, Synthon, and Teva; speaker honorarium from Almirall, Bayer, Biogen, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Sanofi Genzyme, and Teva; and research support from Bayer, Biogen, Novartis, Sanofi Genzyme, and Teva. Elzbieta Jasinska received fees for participation in scientific advisory boards from Biogen and speaker honoraria from Adamed, Allergan, Hoffman-La Roche, Krka, Novartis, Polpharma, and Teva. Mark S. Freedman received research or educational grants from Genzyme Canada; honoraria or consultation fees from Actelion, Bayer, Biogen, Celgene, Chugai, EMD Serono Canada, Hoffman-La Roche, Merck Serono, Novartis, PendoPharm, Sanofi-Aventis, and Sanofi Genzyme; participated as a member in a company advisory board, board of directors, or other similar group for Actelion, Bayer, Biogen, Clene, Hoffman-La Roche, MedDay, Merck Serono, Novartis, and Sanofi-Aventis; and served on a company-sponsored speaker’s bureau for Sanofi Genzyme. Anthony J. Lembo received fees for participation in scientific advisory boards from Bayer, Biogen Idec, Ironwood, Salix, Shire, and Takeda. Krzysztof Selmaj received consulting fees from Genzyme, Novartis, Ono, Roche, Synthon, and Teva, and speaker fees from Biogen. Ilda Bidollari, Richard Leigh-Pemberton, Maria Lopez-Bresnahan, and David Rezendes are employees of and hold stock/stock options in Alkermes. Hailu Chen, Jerome Hanna, Jennifer Lyons, and Catherine Miller are employees of and hold stock/stock options in Biogen. Jerry S. Wolinsky received compensation for consulting, scientific advisory boards, or other activities with AbbVie, Acorda Therapeutics, Alkermes, Brain Cell Therapeutics, Celgene (Exp 11 2019), Clene Nanomedicine, EMD Serono, Forward Pharma, GeNeuro, GW Pharma, MedDay Pharmaceuticals, NervGen Pharma Corp., Novartis, Otsuka, PTC Therapeutics, Roche/Genentech, and Sanofi Genzyme; compensation for CME activities with AcademicCME, PlatformQ Health Education, PRIME, and Strategic Consultants Intl.; and royalties for out-licensed monoclonal antibodies through UTHealth from Millipore Corporation.
Figures

References
-
- Biogen. Vumerity® (diroximel fumarate) prescribing information and patient information. 2019. https://www.vumerity.com/content/dam/commercial/vumerity/pat/en_us/pdf/v.... Accessed 19 Dec 2019.
-
- Biogen. Tecfidera® (dimethyl fumarate) prescribing information and patient information. 2019. https://www.tecfidera.com/content/dam/commercial/multiple-sclerosis/tecf.... Accessed 9 Apr 2019.
-
- Wehr A, Hard M, Yu M, Leigh-Pemberton R, von Moltke L. Relative bioavailability of monomethyl fumarate after administration of ALKS 8700 and dimethyl fumarate in healthy subjects. Neurology. 2018;90(Suppl. 15):P1.403.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

